(From Anscher, above)
In 1997, a consensus panel was convened by the American Society for Therapeutic Radiology
and Oncology (ASTRO) to formulate guidelines for the role of salvage radiotherapy for PSA
failures after radical prostatectomy. No data from randomized trials were available. Based
upon retrospective data, the panel could not identify any subgroup of patients with a
higher or lower success rate from salvage RT. It was felt, however, that early treatment
(i.e., before the PSA rose above 1.5 ng/mL) was more likely to be successful than delayed
therapy. The panel also recommended utilizing doses = 64 Gy.
Our results do not support the
findings of the consensus conference in that we could identify seven factors that had a
significant relationship to DFS. As in our earlier report, pre-RT PSA =2.5 ng/mL was again
a significant predictor of DFS, despite the fact that the composition of the present
series differs somewhat from our original report. Since our previous analysis, we have
been less likely to offer salvage RT to patients with prognostic factors that predict for
the presence of occult distant metastases. This policy is reflected in the more favorable
composition of the present series. For example, compared to our earlier analysis, the
present group has a smaller percentage of patients with seminal vesicle involvement (34%
vs. 49%) or positive nodes (3% vs. 6%). In addition, the median pre-RT PSA of the present
series is lower than in our prior publication (1.4 vs. 2.5 ng/mL), reflecting a tendency
to refer patients earlier on the part of urologists.
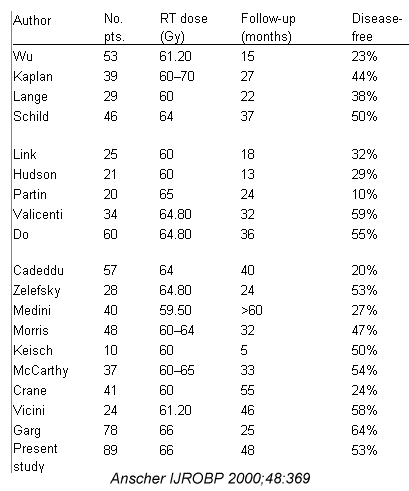
The present
series, however, does support the recommendation of the consensus conference to utilize RT
doses =64 Gy. In fact, the only significant variable related to DFS that we could identify
on multivariate analysis was a RT dose >65 Gy. This finding is consistent with the
report of Schild et al. in which a dose =64 Gy was
positively correlated with DFS. Similar results have been reported by Forman
and Velasco. In contrast, the median dose in our prior report was 61.2 Gy, which may, in
part, be responsible for the poorer results in that series.
Late complications in the present
series, while common, were generally mild. A surprising finding was that the
complication rate among patients treated using 3DRT was higher than in non-3D patients.
There are two possible reasons for this phenomenon. First, the median dose in the 3D group
was higher than in the non-3D group (66 Gy vs. 61 Gy). Second, it is possible that the use
of 3D techniques resulted in the use of larger fields and, therefore, in the inclusion of
larger volumes of bladder and rectum in the high-dose volume. Unfortunately, this latter
question cannot be definitively addressed herein, due to the impossibility of accurately
estimating volumes of irradiated bladder and/or rectum in the non-3D patients. The fact
that the lateral field areas were similar between the 3D and non-3D patients, however,
suggests that this difference in complications may be more related to the use of higher
doses in the 3D group than to the inclusion of a larger volume of normal tissue in the
irradiated fields.
Previous reports have also documented
a low rate of serious late complications. Grade 3/4
genitourinary (GU) complications have been noted to occur in 0–6% of patients.
Similarly, Grade 3/4 gastrointestinal (GI) complications have been described in 0–1%
of patients receiving salvage postprostatectomy RT. Grade 1/2 GU and GI complications have
been described in 0–21% and 2–17% of patients, respectively. Radiation-induced
urinary incontinence has been reported to occur in 4–19% of patients, when
administered after radical prostatectomy (most series report <10%). Recently, it has
been suggested that radiation given in the adjuvant setting after radical prostatectomy,
rather than for salvage of a rising PSA, is associated with a lower incidence of
incontinence. The lower dose used to irradiate patients in the adjuvant setting probably
accounts for this finding.
In summary, our intermediate-term data
suggest that about 70% of patients receiving salvage pelvic RT for a rising PSA will
achieve complete remission. Of those achieving remission, about 75% will remain
disease-free at 4 years. Whether these remissions can be sustained beyond this time point
will require additional follow-up. Doses above 65 Gy should be
utilized. Early intervention, i.e., before the PSA gets much above 1 ng/mL is
advocated.
ASTRO recently (J Clin Onc
1999;17:1155) published Guidelines for using Radiation for elevated PSA after Radical
Prostatectomy and concluded that: radiation will be effective in 70%
(response) but only 27-45% will be cured (have neg. PSA at 5 years); it is probably
reasonable to wait until PSA rises slightly (level of 0.5) but should start XRT before PSA
exceeds 1.5; dose of radiation should be 64Gy, and the role of combining XRT with
hormonal therapy is unclear at this time. Specifically THE STATEMENT READ AS FOLLOWS:
PURPOSE: To develop evidence-based guidelines for (1)
prostate re-biopsy after radiation and (2) radiation therapy with rising prostate-specific
antigen (PSA) levels after radical prostatectomy in the management of patients
with localized prostatic cancer.
DESIGN: The American Society of Therapeutic Radiology and
Oncology (ASTRO) challenged a multidisciplinary consensus panel to address
consensus on specific issues in each of the two topics. Four well-analyzed
patient data sets were presented for review and questioning by the panel. The
panel sought criteria that would be valid for patients in standard clinical
practice as well as for patients enrolled in clinical trials. Subsequent to an
executive session that followed these presentations, the panel presented
its consensus guidelines.
RESULTS AND CONCLUSIONS: Based on the data presented, the
prostate re-biopsy negative rates ranged from 62% to 80% for patients with
stage T1-2 tumors. The panel judged that prostate re-biopsy is not necessary as
standard follow-up care and that the absence of a rising PSA level after
radiation therapy is the most rigorous end point of total tumor eradication.
Further, the panel judged that re-biopsy may be an important research tool.
Based on the data presented, the long-term (5 years or more) PSA remission
rate after salvage radiation therapy ranges from 27% to 45%. The panel
requested results from prospective randomized trials to evaluate optimally this
information. The panel judged that the total dose of
radiation should be 64 Gy or slightly higher and that, in patients with or
without radiation therapy, there is no standard role for androgen suppressant
therapy for rising PSA values after prostatectomy.
CONSENSUS PANEL STATEMENT: CONSENSUS PANEL GUIDELINES FOR
THE ROLE OF SALVAGE RADIOTHERAPY FOR PSA FAILURES AFTER RADICAL PROSTATECTOMY
The panel was asked to reach a consensus, if possible, on
the success of external-beam radiation as salvage therapy to cure patients
with elevated PSA levels after radical prostatectomy, on the complete PSA
remission rate and the durability of this remission after salvage radiation,
the possible identification of subgroups with higher or lower probability of
benefit, and the identification of optimal timing of salvage irradiation.
The rate of a complete biochemical remission after
radiation therapy certainly depends on which patients are selected
for this treatment. From the presented data, approximately 70% of
treated patients had a complete PSA remission. The panel was unwilling
to come to consensus now on the durability of a complete PSA
remission after irradiation. The data presented from the four
invited institutions ranged in median follow-up from 25 months to 67
months, and the actuarial biochemical failure-free survival rates at
5 years ranged from 27% to 45%. This range was judged to be an
interim but an uncertain alternative to the results from
prospective, randomized trials that are nearing completion or that
are under way to evaluate optimally this information.
• If adjuvant or postoperative radiation treatment has been
withheld because the pathology findings indicated local cure was
likely, then waiting for secure evidence of a PSA failure (rising to
a level of 0.5 ng/mL) likely does not decrease the probability of a
patient being secondarily cured. In this instance clinicians can be
more confident that the patient actually has relapsed disease and
warrants treatment if they wait until PSA levels rise to this
clearly measurable level. The panel noted that most data indicate
the PSA level at time of salvage radiation may make a prognostic
difference in outcome. The PSA level or threshold, based on the data
presented, seemed to be 1.5 ng/mL.
• There are presently no specific subgroups of patients, based
on either the pathologic information from the prostatectomy specimen
or the preprostatectomy PSA levels, who indicate a higher or lower
success rate with salvage radiation therapy.
• The highest dose of radiation therapy that can be given without
morbidity is justifiable. Based on the data presented at this
conference, the dose should be 64 Gy or slightly higher with
standard fractionation (1.8 or 2.0 Gy per fraction).
• There is no standard role for androgen suppression therapy in
patients with or without radiation therapy for rising PSA values
after prostatectomy. The panel judged that the use of hormonal
therapy in this setting is still investigational. As such, a
cautious approach to the wholesale use of hormone therapy could help
reduce cost in the managed-care setting. |