|
Side Effects of
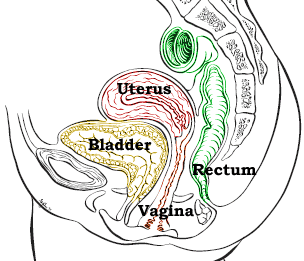
Pelvic Radiation for Gynecological Cancers Note that radiation can be external or internal or HDR. The side effects of radiation are related to: the area treated (which normal organs are in the way, e.g. the bowel, bladder or rectum, see CT picture of organs affected by radiation here) the size of the radiation field or port (the larger the area, the more the side effects) the dose of radiation (the higher the dose, the more side effects) and whether chemotherapy is being given as well (generally more fatigue and diarrhea). For a list of the most likely side effects from gynecologic radiation read the consent forms from the RTOG trials here. Read the nursing instruction sheets here. |