|
External beam radiation therapy for squamous cell carcinoma of the soft palate.
Medini E, Medini A,
Int J Radiat Oncol Biol Phys 1997 Jun 1;38(3):507-11
Department of Radiation Oncology and Otolaryngology, University of Minnesota, and
Veterans Administration Medical Center, Minneapolis 55417, USA.
A total of 24 patients with squamous cell carcinoma of the soft palate were treated at
the Veterans Administration Medical Center Minneapolis, MN, between February 1977 and May
1992. Of the 24 patients 2 had T1, 19 T2, 1 T3, and 2 had T4 lesions. Nineteen patients
did not have clinical nodal disease, stage (N0), 1 had N1, 2 N2, and 2 N3 disease (Table
1). All the patients were treated by 4 MeV linear accelerator. A 1.75 Gy median dose was
administered per fraction to a total of 70 Gy median dose.
Bilateral opposed compensated shrinking fields technique was used. RESULTS: The 3-year
disease free survival rate after external beam radiation therapy was
100%
(1 out of 1), 64.7% (11 out of 17), 100% (1 out of 1), and 0%, for patients with T1, T2,
T3, and T4 disease, respectively. Salvage surgery for recurrent disease was
successful in 57.1% (4 out of 7 patients (Table 2). The ultimate 3-year disease free
survival rate for the entire group, including surgical salvage, was 81% (17 out of 21)
(Fig 1). CONCLUSION: Radiation therapy alone in our institution resulted in tumor control
and survival rates compare favorably to previously published reports in the literature.
Surgery can be reserved as salvage procedure.
Results of irradiation in squamous cell carcinoma of the soft palate and uvula.
Keus RB, Pontvert D, Brunin F, Jaulerry C, Bataini JP
Radiother Oncol 1988 Apr;11(4):311-7
Radiotherapy Department, The Netherlands Cancer Institute (Antoni van Leeuwenhoekhuis),
Amsterdam.
Out of a series of 235 patients presenting with tumours of the soft palate at the
Institut Curie, between 1958 and 1980, 146 cases were analysed to evaluate the results of
radical radiation therapy. Seventy patients (48%) had advanced T3-T4 disease and 40
patients (27%) had clinically involved metastatic nodes. All patients had a minimum
follow-up of 5 years. In 103 cases, megavoltage X-ray therapy was employed. For 43
patients, presenting with small or moderately advanced tumours, a combination of
megavoltage and intra-oral orthovoltage X-rays was used.
The local control rate at 3 years
was 92% for T1, 70% for T2, 58% for T3 and 49% for T4 lesions. Nodal failure was seen in 19 patients. In 9 of these, it was not
associated with failure at the primary site, 7 out of 9 occurring marginally or outside
the treatment portals. Complications were observed in 16 patients, 7 requiring surgery.
The crude 3 and 5 year survival rate was 52 and 40%, respectively, and the disease-free
survival 59 and 53%.
Primary radiation therapy in the treatment of squamous cell carcinoma of the soft
palate.
Horton D, Tran L, Greenberg P, Selch MT, Parker RG. Cancer 1989 Jun 15;63(12):2442-5
Department of Radiation Oncology, School of Medicine, University of California Los
Angeles.
From 1970 to 1986, 45 patients received primary radiation therapy for squamous cell
carcinoma of the soft palate at the University of California Los Angeles (UCLA) Center for
the Health Sciences and Wadsworth Veteran's Administration Hospital. Seven patients were
lost to follow-up or had prior irradiation, and were excluded. Thirty-eight patients
received a median dose of 70 Gy (62.5 to 80 Gy) to the primary site. After a median
follow-up of 48 months, initial control of disease at the primary site was accomplished in
74% of the patients. The initial control by stage was as follows:
TI, 83%, T2, 67%; and T3, 63%. After surgical salvage, local control increased to 92%, 80%
and 75% for stages T1, T2, and T3, respectively. Twenty-nine percent (11 of 38)
of the patients had cervical node metastases at presentation. Radiation provided regional
control in 96% (26 of 27) with N0 disease and 86% (six of seven) with N1 disease at
diagnosis. Sixteen patients (42%) had an additional malignancy of the upper aerodigestive
tract. Because the incidence of second malignancies after treatment is high and surgical
salvage of treatment failures is possible, close follow-up is essential in the management
of this tumor. We conclude that radiation therapy is an effective modality for the
treatment of squamous cell carcinoma of the soft palate. Primary radiation therapy may
offer many patients the chance to avoid surgical procedures that are both cosmetically and
functionally debilitating without compromosing treatment outcome.
Carcinoma of the soft palate treated with irradiation: analysis of results and
complications.
Amdur RJ, Mendenhall WM, Parsons JT, Isaacs JH Jr, Million RR, Cassisi NJ. Radiother Oncol 1987 Jul;9(3):185-94
This is an analysis of 75 patients with squamous cell carcinoma of the soft palate
and/or uvula treated with radical radiation therapy alone (64) or in conjunction with
planned neck dissection (11) between October 1964 and September 1983. All patients have a
minimum follow-up of 2 years and 60 (80%) have a minimum follow-up of 5 years. Patients
were excluded from analysis of disease control at the primary site and/or neck if they
died within 2 years of treatment with the site(s) continuously disease free.
The initial local control rates and
ultimate local control rates after surgical salvage of irradiation failures
for patients treated with continuous-course irradiation were as follows: T1,
8/8 (100%) and 8/8 (100%); T2, 14/19 (74%) and 16/19 (84%); T3, 5/11 (45%)
and 5/11 (45%); and T4, 1/4 (25%) and 1/4 (25%). Overall,
7/55 patients (13%) treated with continuous-course irradiation experienced
irradiation-related bone or soft tissue complications; there was only one severe
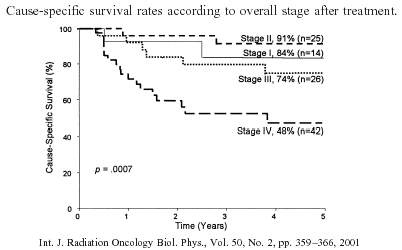
complication. The 5-year determinate survival rates by modified AJCC stage for patients
treated with continuous-course irradiation are as follows: I, 83%; II, 78%; III, 38%; IVA,
0/2; and IVB, 25%
Primary radiation therapy in
the treatment of squamous cell carcinoma of the soft palate
|
| David Horton, MD |
1Department of Radiation
Oncology, School of Medicine, University of California Los Angeles,
Los Angeles, California and the Jonsson Comprehensive Cancer Center
2Wadsworth Veteran' Administration Medical Center,
Department of Radiation Oncology, West Los Angeles, California
|
| From 1970 to 1986, 45 patients
received primary radiation therapy for squamous cell carcinoma of
the soft palate at the University of California Los Angeles (UCLA)
Center for the Health Sciences and Wadsworth Veteran' Administration
Hospital. Seven patients were lost to follow-up or had prior
irradiation, and were excluded. Thirty-eight patients received a
median dose of 70 Gy (62.5 to 80 Gy) to the primary site.
After a median follow-up of 48 months, initial control of disease at
the primary site was accomplished in 74% of the patients.
The initial control by stage
was as follows: T1, 83%; T2, 67%; and T3, 63%. After surgical
salvage, local control increased to 92%, 80%, and 75% for stages T1,
T2, and T3, respectively. Twenty-nine percent (11 of 38) of
the patients had cervical node metastases at presentation. Radiation
provided regional control in 96% (26 of 27) with NO disease and 86%
(six of seven) with N1 disease at diagnosis. Sixteen patients (42%)
had an additional malignancy of the upper aerodigestive tract.
Because the incidence of second malignancies after treatment is high
and surgical salvage of treatment failures is possible, close
follow-up is essential in the management of this tumor. We conclude
that radiation therapy is an effective modality for the treatment of
squamous cell carcinoma of the soft palate. Primary radiation
therapy may offer many patients the chance to avoid surgical
procedures that are both cosmetically and functionally debilitating
without compromising treatment outcome. |
|