Definitive
radiotherapy is uncommonly used in the management of soft-tissue sarcoma
(STS). The purpose of the study was to evaluate the results of
radiotherapy for unresected STSs treated in a single institution.
Methods and
Materials: Between 1970 and 2001, 112 patients with STSs underwent
radiotherapy for gross disease. Locations of the tumor were 43% in the
extremities, 26% retroperitoneal, 24% in the head and neck, and 7% in
the truncal wall. Histologic grades were 11% G1 and 89% G2 to G3. Median
size of tumor at radiotherapy was 8 cm (range, 1–30 cm). Median
radiation dose was 64 Gy (range, 25–87.5 Gy). Twenty percent of patients
received chemotherapy. Local control (LC), disease-free survival (DFS),
and overall survival (OS) rates were evaluated.
Median
follow-up for patients is 139 months (range, 30–365 months).
The 5-year actuarial LC, DFS,
and OS were 45%, 24%, and 35%, respectively.
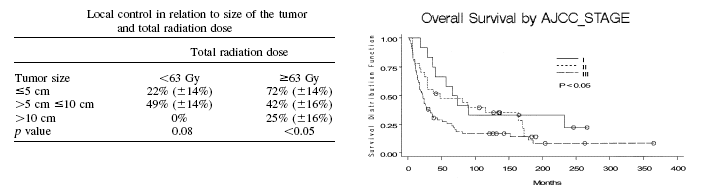
Tumor size at radiotherapy and radiation dose influenced LC, DFS, and OS
in univariate analysis. LC at 5
years was 51%, 45%, and 9% for tumors less than 5 cm, 5 to10 cm, and
greater than 10 cm, respectively.
Patients who received doses of
less than 63 Gy had 5-year LC, DFS, and OS rates of 22%, 10%, and 14%,
respectively, compared with 5-year LC, DFS, and OS rates of 60%, 36%,
and 52%, respectively, for patients who received doses of 63 Gy
or more. Use of chemotherapy, histologic grade, age, and location did
not influence results. Major
radiotherapy complications were noted in 14% of patients; 27% of
patients who received doses of 68 Gy or more had these complications
compared with 8% of patients treated with doses of less than 68 Gy.
Conclusions
Definitive radiotherapy for STS should be considered in clinical
situations where no acceptable surgical option is available. Higher
radiation doses yield superior tumor control and survival. A rise in
complications occurs in patients who receive doses of 68 Gy or more,
which provides a therapeutic window for benefit in these patients. When
we take into account a dose–response effect in our study and a
relationship of dose and occurrence of serious complications,
we see that doses in the range
of 63 to 67 Gy for gross disease of STS are able to control a
significant proportion of patients, with lower complication rates than
that expected for doses greater than 68 Gy. We did not study the
influence of details of radiation techniques beyond the dose on the
occurrence of complications. The possibilities of further safe dose
escalation by use of more advanced radiotherapy technologies such as
conformal radiotherapy, IMRT, and proton therapy remain an open
question. We did not find any favorable effect of chemotherapy on
treatment outcomes. Chemotherapy given for STS in adjuvant settings
offer possible modest improvement of 10-year overall survival of 7%,
according to the most recent Cochran meta-analysis. Combined
radiochemotherapy is more effective in inducing responses than any
modality alone; however, in the study by Spunt the better response
to combined treatment was not related to improved survival. The
significant toxicity of the combined approach was reported, and such a
treatment could be difficult to deliver for many patients from our study
because of their advanced age and comorbidity. Our results show that a
substantial proportion of this unfavorable population of patients,
however, could still be cured with definitive radiotherapy.