| To report mature results of a randomized trial that compared
extended-field radiotherapy (EFRT) versus pelvic radiotherapy with
concomitant fluorouracil and cisplatin (CTRT) in women with
locoregionally advanced carcinomas of the uterine cervix.
PATIENTS AND
METHODS: Four hundred three women with cervical cancer were randomly assigned
to receive either EFRT or CTRT. Patients were eligible if they had stage IIB to IVA disease, stage IB to IIA disease with a
tumor diameter  5 cm, or
positive pelvic lymph nodes. Patients were stratified by stage and
by method of lymph node evaluation. 5 cm, or
positive pelvic lymph nodes. Patients were stratified by stage and
by method of lymph node evaluation.
RESULTS: The median follow-up time for 228 surviving patients was 6.6 years.
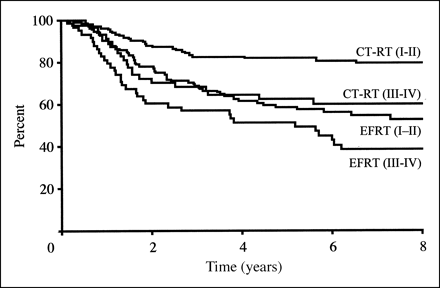
The overall survival rate for patients treated with CTRT was
significantly greater than that for patients treated with EFRT (67% v
41% at 8 years). There was an overall reduction in the risk of
disease recurrence of 51% for patients who received CTRT. The rate of serious late
complications of treatment was similar for the two treatment arms.
Treatment
External-beam radiotherapy was delivered with anteroposterior-posteroanterior opposed
beams of at least 15-MV photons or with four fields (anteroposterior,
posteroanterior, and two lateral fields) of at least 4-MV photons. For patients
who were assigned to receive radiotherapy and chemotherapy, the treatment
field extended from the space between L4 and L5 to the midpubis or to a line
4 cm below the most distal vaginal or cervical site of disease. Lateral
fields were designed to encompass S3 posteriorly, with a margin of at least 3
cm from the primary cervical tumor. Custom shielding was designed to treat the
pelvic lymph nodes, with a margin of at least 1 to 1.5 cm. For patients who
were assigned to receive radiotherapy alone, the pelvic and para-aortic areas
were treated as a continuous area, with a superior field border at the
space between L1 and L2. The radiation dose was calculated at the patient's
midplane in the central ray of the field (for anteroposterior-posteroanterior
fields) or to the isocenter of the beams. The total dose to be delivered to
the pelvic and para-aortic lymph nodes was 45 Gy, given at a dose of 1.8 Gy
per fraction.
Three hundred seventy-seven of 388 patients with available information were
treated with a combination of external-beam and intracavitary therapy. In 315
patients, the first intracavitary treatment was delivered after 45 Gy of
external radiotherapy had been delivered to pelvic or pelvic and para-aortic
nodes. In 72 patients, the first intracavitary treatment was performed after 20
to 30 Gy of pelvic external-beam radiotherapy and additional external-beam
therapy was delivered with a midline block. Interstitial brachytherapy was
used only if necessary to increase the dose directed at distal vaginal sites of
disease. Brachytherapy was performed within 2 weeks (preferably within 1 week)
after the completion of pelvic radiotherapy, with the goal of keeping the total
duration of treatment less than 8 weeks when possible. The protocol
specified that all patients receive a total cumulative dose to point A (a
reference location 2 cm lateral and 2 cm superior to the cervical os) of at
least 85 Gy. The suggested maximal doses to the bladder, the rectum, and the
lateral surface of the vagina were 75, 70, and 130 Gy, respectively.
It was recommended that the total course of treatment be completed in less than
56 days.
Within 16 hours after the first radiation fraction was administered, patients
in the combination-therapy group received the first cycle of chemotherapy,
which consisted of an IV infusion of cisplatin 75 mg/m2
of body-surface area over a 4-hour period followed by an IV infusion of
fluorouracil 4,000 mg/m2 over a 96-hour period. Thus, chemotherapy
was administered during days 1 through 5 of radiotherapy. Two additional cycles
of chemotherapy were scheduled at 3-week intervals. One of these was
administered at the time of the second intracavitary insertion. Patients who
developed a WBC count less than 1,500/ÁL, an absolute granulocyte count less
than 1,000/ÁL, a platelet count less than 75,000, or grade 4 nausea or
diarrhea had treatment interrupted for up to 1 week followed by a 50% reduction
in the dose of fluorouracil. If these side effects persisted for more
than 1 week, no additional fluorouracil was given. |