| After radiation the PSA slowly
declines. The low point or nadir may not be reached for several
years. Rather than continuously declining, occasionally the PSA may
temporarily rise which is referred to as a PSA bounce. PSA bounce is
a common phenomenon after prostate brachytherapy and occurs at a
rate of 17–31%, depending on the definition used. It is more common
in younger patients, those receiving higher implant doses, and those
with larger glands. PSA bounce does not predict for future PSA
failure. Prostate-specific antigen bounce after prostate seed implantation for localized prostate cancer: descriptions and implications Richard G Stock, Nelson N Stone, Jamie A Cesaretti International Journal of Radiation Oncology * Biology * Physics 1 June 2003 (Vol. 56, Issue 2, Pages 448-453) To calculate the actuarial risk of developing a prostate-specific antigen (PSA) bounce after prostate brachytherapy alone, using three definitions of bounce mentioned in the literature, and to explore the relationship between disease and treatment variables and the risk of developing a bounce. The impact of PSA bounce on PSA failure was also explored. Prostate-specific antigen (PSA) is a sensitive measure of treatment outcome after radiotherapy (RT) for prostate cancer. Unlike after radical prostatectomy, when PSA should be undetectable after treatment, the PSA level after RT falls slowly and may intermittently increase over a number of years. This may be due to PSA release from partially damaged normal prostatic epithelium and/or the long time it may take for lethally damaged cancer cells to die and stop producing PSA. This is especially relevant for prostate brachytherapy, in which it takes 2–10 months for the seeds to deliver their entire radiation dose. Because of the nature of PSA decline after RT, the American Society for Therapeutic Radiology and Oncology (ASTRO) created guidelines for biochemical failure that used three consecutive elevations of PSA to signal failure. Elevations in PSA that occur months to years after treatment and that subsequently fall can confound this definition and make it difficult to distinguish an actual failure from what has been described as a benign PSA bounce. Various definitions of PSA bounce have been used. Critz defined a PSA bounce as a rise of 0.1 ng/mL after brachytherapy and external beam RT (EBRT). Cavanagh described a temporary increase ≥0.2 ng/mL. Pruthi believed that a PSA bounce should be defined as a rise in PSA of >35% from the baseline prior value. This opinion was based on the work of Prestigiacomo and Stamey who noted that overall PSA variability was 35% in patients without prostate cancer. This variability was composed of physiologic and assay variation. Hanlon defined a PSA bounce after EBRT as a rise of 0.4 ng/mL during a 6-month period. The level and significance of a bounce may also be different depending on the type of RT delivered. The current study examined PSA bounce after brachytherapy alone without hormonal therapy or EBRT. The incidence of PSA bounce was calculated using three different definitions (≥0.1 ng/mL, ≥0.4 ng/mL, and >35%). In addition, the effects of pretreatment disease and treatment-related factors on PSA bounce were analyzed to determine which patients were at increased risk of developing a bounce. Finally, the relationship of PSA bounce to biochemical failure was explored. Methods and materials A total of 373 patients with T1–T2 prostate cancer underwent radioactive seed implant using 125I (n = 337) or 103Pd (n = 36) without hormonal therapy or external beam RT. All patients had a minimum of 1 year (median 4, maximum 11) of follow-up and at least three follow-up PSA values. PSA bounce was defined by a rise of one or two PSA values with a subsequent fall. Three definitions of bounce were used: definition 1, rise ≥0.1 ng/mL; definition 2, rise ≥0.4 ng/mL; and definition 3, rise >35% of previous value. Results The actuarial likelihood of experiencing a PSA bounce at 5 years was 31% for definition 1, 17% for definition 2, and 20% for definition 3. The median time to develop a bounce was 19.5 months for definitions 1 and 2 and 20.5 months for definition 3. Gleason score, initial PSA level, and clinical stage did not predict for bounce using any definition. Using definition 1, younger patients (≤65 years) had a bounce rate at 5 years of 38% vs. 24% for older patients (p = 0.009). 125I patients receiving an implant dose of ≤160 Gy had a bounce rate (definition 1) at 5 years of 24% vs. 38% for those receiving a dose delivered to 90% of the gland on the 1 month postimplant dose–volume histogram (D90) >160 Gy (p = 0.04). Using definition 2, prostate volume significantly affected the incidence of bounce. Patients with larger glands (>35 cm3) were more likely to experience a bounce (23% at 5 years) than those with smaller glands (≤35 cm3) who had a bounce rate of 11% at 5 years (p = 0.01). In a multivariate analysis of factors predicting for PSA failure, PSA bounce was not found to be significant. Discussion The recognition of PSA bounce is important in treating patients who receive RT for prostate cancer. Unlike radical prostatectomy, with which the PSA level should fall to undetectable levels, RT, and especially brachytherapy, cause a gradual decline in PSA. PSA values may take as long as 4–5 years to reach a nadir. During this time, fluctuations in PSA levels may cause great anxiety for patients. The intact prostate, with its normal and abnormal epithelial cells, is the source for these PSA fluctuations. A transient rise in PSA has been recognized since PSA began to be used as a tool to monitor disease progress. This PSA bounce was mentioned in the original ASTRO consensus statement that provided guidelines for monitoring PSA levels after RT and was one of the reasons that three consecutive PSA rises were used to define failure. Various definitions of PSA bounce have been cited in the literature. In the current report, three reported definitions (rise of ≥0.1, ≥0.4, and >35% of baseline) were used to calculate the actuarial bounce rate after brachytherapy. A comparison of these rates demonstrated that definition 1 would lead to a higher bounce rate (31%) than definition 2 (17%) or 3 (20%) at 5 years. The rate of 31% is similar to the 35% rate reported by Critz after implant and EBRT using the same definition. In addition, Cavanagh also noted a PSA bounce (≥0.2 ng/mL) in 35.8% of patients treated with brachytherapy with or without EBRT. Definition 2 led to a lower actuarial bounce rate of 17% at 5 years compared with the bounce rate after EBRT reported by Hanlon of 31%. Of note, all the above-mentioned studies used crude rates to estimate the risk of bounce, and the current study used actuarial methods, which more accurately estimate the risk of developing a bounce over time. The definition proposed by Pruthi (>35%) led to a bounce rate of 20%, similar to the rate calculated using definition 2. Because patients become concerned with any rise in their PSA level, all definitions are equally valid, unless one is associated with a greater likelihood of developing PSA failure. In the current series, PSA bounce (using all three definitions) did not predict for PSA failure. In a multivariate analysis of potential predictors of PSA failure, bounce was not found to be a significant predictor. This is similar to the findings reported by Critz. Of note, using definition 1, PSA bounce predicted for PSA control. An explanation for this is that patients with a PSA bounce (definition 1) were more likely to have received a higher dose implant, and higher dose implants are associated with lower PSA failure rates. This explanation is supported by the multivariate analysis of PSA failure, which revealed dose, but not bounce, to be a significant predictor. This contrasts with the findings of Hanlon who found that patients developing a bounce had a significantly lower biochemical disease-free rate (47%) compared with those who did not (66%). This suggests that the bounce phenomenon may be different after EBRT than after brachytherapy. The ability of prognostic variables to predict for a bounce varied depending on the definition used. Calculations with definition 1 revealed that both younger age (<65 years) and greater implant dose (>160 Gy for 125I) were associated with an increased incidence of bounce (38% at 5 years). The effect seen with age may be secondary to increased testosterone levels, although this was not measured in the current study, or more reactive epithelial cells. The increased rate of bounce seen with higher doses may be related to a greater likelihood of a radiation-induced inflammatory reaction. This contrasts with the findings of Hanlon that showed that patients with a bounce were treated to lower median dose than those without a bounce. This again suggests that PSA bounce after EBRT may be different and have different implications than the bounce seen after brachytherapy. The cause of this PSA bounce is unknown, but the timing of its occurrence suggests that it may be related to a late radiation reaction. The median time to bounce ranged from 19.5 to 20.5 months using all three definitions. This time to bounce is similar to the time to develop radiation proctitis and erectile dysfunction A similar finding was reported in the study by Critz in which the median time to bounce was 18 months after implant. These findings differ from those of Hanlon, who noted a median time to bounce of 35 months. It is also important to note that a bounce occurred as late as 88 months after implant. Unlike the report from Critz, in which the PSA bounce was not seen in patients treated with combined implant and EBRT after 5 years, patients treated with implant alone can experience a transient rise in PSA as long as 9 years after treatment. PSA bounce is a common phenomenon after brachytherapy and occurs at a rate of 17–31%, depending on the definition used. The similar findings of the current report to those found by Critz suggest that the mechanism for this bounce is the same whether the patient receives an implant alone or in combination with EBRT. On the other hand, it appears that PSA bounce after brachytherapy may be different from that seen after EBRT. The lower rate of bounce, earlier time to bounce, differing effect of dose, and lack of effect on PSA failure compared with the phenomena seen after EBRT suggest that they may be two different types of PSA profiles. The data after EBRT suggest that PSA bounce may represent an early manifestation of PSA failure in a certain percentage of patients and the phenomena seen after brachytherapy seem to have little effect on PSA failure developing. All this information can help aid physicians in providing counsel to patients during their postbrachytherapy follow-up period. Conclusion PSA bounce is a common phenomenon after prostate brachytherapy and occurs at a rate of 17–31%, depending on the definition used. It is more common in younger patients, those receiving higher implant doses, and those with larger glands. PSA bounce does not predict for future PSA failure.
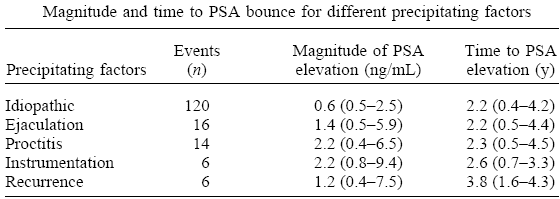
Using the magnitude of PSA
bounce after MRI-guided
prostate brachytherapy to distinguish recurrence, benign
precipitating factors, and idiopathic
bounce
To identify events that precipitated a prostate-specific antigen (PSA) bounce and characterize the magnitude, duration, and time to PSA bounce after MRI-guided prostate brachytherapy. Between 1997 and 2001, 186 patients with low-risk prostate cancer underwent MRI-guided permanent 125I source implantation, with or without external beam radiotherapy. A PSA bounce was defined as a ≥15% elevation in PSA compared with the most recent value, followed by a decline to a level at or less than the prebounce value. At the time of PSA measurement, data were prospectively collected on whether the patient had recent ejaculation, ongoing radiation proctitis, or recent instrumentation. Brachytherapy has become a frequently used treatment for early-stage prostate cancer. Although the long-term outcome after brachytherapy for early-stage prostate cancer remains to be determined, a recent study with up to 12 years of follow-up reported excellent long-term disease control in a cohort of patients treated with brachytherapy. Another study demonstrated similar 5-year outcomes in low-risk prostate cancer patients treated with brachytherapy compared with those treated with radical prostatectomy or external beam radiotherapy (EBRT). Serum prostate-specific antigen (PSA) levels are used as a measure of disease recurrence after brachytherapy, EBRT, and radical prostatectomy. After radical prostatectomy, the PSA level declines to undetectable levels within weeks. However, after RT, PSA remains detectable and falls more slowly, because the prostate has not been removed. Hence, elevations in PSA have been used as a marker for recurrence after RT. The American Society for Therapeutic Radiology and Oncology (ASTRO) Consensus Panel has defined biochemical failure as three consecutive rises in PSA after RT for prostate cancer. In addition to PSA elevations from disease recurrence, prostate cancer patients treated with RT can also show a temporary elevation in PSA without disease recurrence, a phenomenon termed PSA bounce. PSA bounce has previously been defined as an increase of ≥0.1 ng/mL followed by a subsequent decrease to less than that level; as an increase of ≥0.2 ng/mL followed by a decline; or as a minimal rise of 0.4 ng/mL during a 6-month period followed by a drop of any magnitude. PSA bounce is a very common phenomenon in prostate cancer patients treated with brachytherapy or EBRT. It has been reported in 24–36% of patients treated with brachytherapy with or without EBRT and in 31% of patients treated with EBRT alone. The etiology of PSA bounce remains unclear. Bacterial and radiation prostatitis have been postulated as possible mechanisms underlying PSA bounce. Previous studies have also shown that transient elevations in PSA can arise from recent ejaculation, instrumentation, and bicycle riding. The PSA bounce phenomenon can complicate the follow-up of prostate cancer patients treated with brachytherapy. Clinicians may find it difficult to distinguish a PSA bounce from disease recurrence, resulting in unnecessary biopsies or salvage therapy. PSA bounce may also generate considerable anxiety among patients. The purpose of this study was to characterize the etiology, magnitude, and time course of PSA bounce to establish whether the PSA kinetics after MRI-guided prostate brachytherapy can differentiate between PSA bounce and disease recurrence. : A total of 115 patients (61.8%) had a total of 156 PSA bounces. Of these, 36 patients had PSA bounces associated with ejaculation, proctitis, or instrumentation, and 79 experienced idiopathic PSA bounces (not associated with a precipitating event). The magnitude of the PSA bounce was significantly lower for the idiopathic PSA bounce (0.6 ng/mL) compared with that associated with ejaculation (p = 0.003), proctitis (p <0.0001), or instrumentation (p = 0.007). Patients with biopsy-proven local recurrence had a median PSA elevation of 1.2 ng/mL, significantly higher (p = 0.006) than the magnitude of the idiopathic PSA bounce, but not significantly different from the magnitude of the PSA bounce due to ejaculation, proctitis, or instrumentation. In patients treated with MRI-guided prostate brachytherapy, recent ejaculation, instrumentation, or ongoing radiation proctitis can cause a transient increase in PSA, the magnitude of which is significantly higher than that for idiopathic PSA bounce, but is similar to that in patients with recurrent disease. Patients with prostate cancer treated with RT can experience a temporary elevation in serum PSA without disease recurrence. This phenomenon, called a PSA bounce, occurs commonly in prostate cancer patients treated with brachytherapy. A PSA bounce can be difficult to distinguish from disease recurrence, leading to a diagnostic dilemma for clinicians and anxiety among patients. No universally accepted definition exists for PSA bounce. Previous studies have defined PSA bounce with cutpoints of 0.1 ng/mL, 0.2 ng/mL, and 0.4 ng/mL. However, these definitions may be confounded by the 15% assay variability in commercially available assays for serum PSA. To avoid classifying assay fluctuations as a PSA bounce, we defined PSA bounce as a ≥15% elevation in serum PSA compared with the most recent value, followed by a decline to a level at or less than the prebounce value. Even with this more stringent definition, 61.8% of the patients experienced one or more PSA bounces. The incidence of PSA bounces in our study was higher than the 24–36% rate reported in previous studies. Multiple factors may have contributed to this difference. The clinical characteristics of the patients, such as age, may have differed from those in previous studies. The follow-up was twice as frequent compared with a previous study and may have led to the detection of a higher number of bounces. In our study, most of the peripheral zone received a dose escalation to 150% of the minimal peripheral dose and the transition zone received the minimal peripheral dose or slightly less at the anterior base of the prostate. This technique may have caused a higher incidence of radiation proctitis, resulting in a higher frequency of PSA bounces. The median time to the PSA bounce was longer than the median time of 18–20.4 months reported in previous studies, and this difference may also have been due to variations in patient characteristics, treatment technique, and follow-up. The etiology for PSA bounce remains unclear, although bacterial and radiation proctitis have been postulated as possible mechanisms. In the present study, information was prospectively collected about possible precipitating factors for the PSA bounce. Recent ejaculation, recent instrumentation, and ongoing radiation proctitis accounted for 23% of the PSA bounces. Although previous studies have shown that ejaculation and instrumentation can cause transient elevations in PSA, ours is the first study to show that ongoing radiation proctitis can cause a temporary PSA increase. Of particular importance was the finding that the magnitude of PSA bounce was significantly lower for idiopathic PSA bounces compared with that associated with ejaculation, instrumentation, and proctitis. Distinguishing the PSA bounce from disease recurrence presents a challenge to clinicians. Ours is the first study to indicate that the magnitude of PSA elevation may help distinguish a PSA bounce from recurrence. Patients with an idiopathic PSA bounce had a significantly lower median PSA elevation than did patients with biopsy-proven local recurrence. In contrast, no significant difference was found in the median PSA elevation in patients with a PSA bounce associated with precipitating factors compared with those with recurrence. Therefore, after ruling out precipitating factors, the magnitude of the PSA elevation may help distinguish an idiopathic PSA bounce from recurrence. However, the overlap in the range of PSA elevations due to idiopathic bounce and recurrence was considerable. Although higher PSA elevations (>2.5 ng/mL) occurred only in patients with recurrence and not in those with an idiopathic bounce, lower PSA elevations occurred in both groups. The present study was limited by the short median follow-up of 2.25 years. With longer follow-up, more patients may develop disease recurrence, perhaps including some of the patients now thought to have had a PSA bounce. Longer follow-up is necessary to characterize this phenomenon of PSA bounce fully. The study was also limited by the small number of recurrences (n = 6), which makes it difficult to draw firm conclusions about differences in PSA kinetics between an idiopathic PSA bounce and disease recurrence. Despite the small number of recurrences, the magnitude of PSA elevation was significantly higher for patients with recurrence than for those with idiopathic PSA bounces. Future studies with larger number of recurrences can evaluate this difference in PSA elevation more definitively. In addition, future studies are planned to evaluate whether the prebounce PSA value or the PSA nadir can predict for eventual biochemical recurrence. Because PSA bounce commonly occurs in prostate cancer patients treated with brachytherapy, clinicians should make their patients aware of this phenomenon to forestall anxiety. When a prostate cancer patient treated with brachytherapy presents with an elevation in PSA, a detailed history should be obtained to evaluate for precipitating factors. If precipitating factors are ruled out, the magnitude of the PSA elevation may help distinguish idiopathic PSA bounce from disease recurrence, thus helping clinicians faced with this difficult diagnostic dilemma. |