Long-term
results with immediate androgen suppression and external irradiation in patients with
locally advanced prostate cancer (an EORTC study): a phase
III randomised trial.
Bolla M, Lancet 2002 Jul 13;360(9327):103-6. University Hospital, Grenoble, France
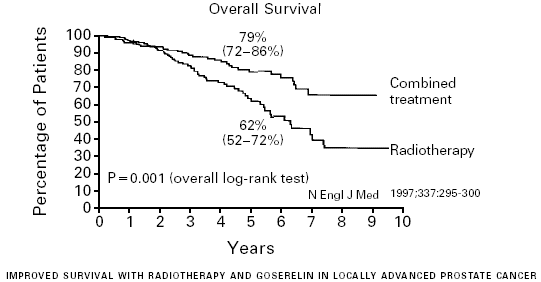
We did a randomised phase III trial comparing external
irradiation alone and external irradiation combined with an analogue of
luteinising-hormone releasing hormone (LHRH) to investigate the added value of long-term
androgen suppression in locally advanced prostate cancer. Between 1987 and 1995, 415
patients were randomly assigned radiotherapy alone or radiotherapy
plus immediate androgen suppression. Eligible patients had T1-2 tumours of WHO
grade 3 or T3-4 N0-1 M0 tumours; the median age of participants was 71 years (range
51-80).
In both treatment groups, 50 Gy radiation was delivered to the pelvis over 5 weeks, and 20
Gy over 2 weeks as a prostatic boost. Goserelin (3.6 mg subcutaneously every 4 weeks) was
started on the first day of irradiation and continued for 3 years;
cyproterone acetate (150 mg orally) was given for 1 month starting 1 week before the first
goserelin injection. The primary endpoint was clinical disease-free survival. Analyses
were by intention to treat.
FINDINGS: 412 patients had evaluable data, with median follow-up of 66 months (range
1-126).
|