Carcinoma of Unknown Primary Site: Sequential Treatment with
Paclitaxel/Carboplatin/Etoposide and Gemcitabine/Irinotecan: A Minnie Pearl Cancer
Research Network Phase II Trial
F. Anthony Greco The
Oncologist, Vol. 9, No. 6, 644-652, November 2004
Since 1995, The Minnie Pearl Cancer Research Network (MPCRN) has performed
five sequential phase II studies in a total of 396 patients with unknown
primary carcinoma using several new chemotherapy combinations. Initially,
paclitaxel (Taxol) was tested in combination with carboplatin (Paraplatin), and
oral etoposide (VePesid) subsequently, docetaxel (Taxotere)
was tested in combination with cisplatin (Platinol) and later with carboplatin. Patients
with favorable prognostic features, known to be more treatable with
better prognoses, were excluded from these studies. After a minimum follow-up
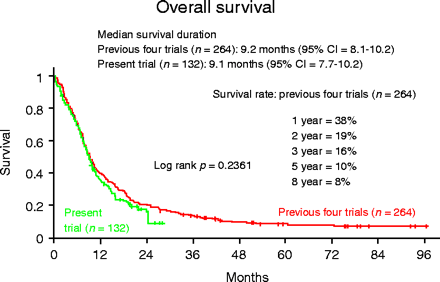
of 4.5 years for the 144 patients in the first three studies, the median
survival time was 10 months and the 1-, 2-, 3-, 4-, 5-, and 8-year survival
rates were 42%, 22%, 18%, 16%, 12%, and 10%, respectively. Gemcitabine
(Gemzar), a drug with activity in several solid tumors, was also found to be
useful as secondary therapy for some patients with carcinoma of unknown primary
site. Subsequently, the combination of gemcitabine, carboplatin, and paclitaxel
in the fourth study in 120 patients was found to be active, to be associated
with acceptable toxicity, and to result in survival similar to our previous
three taxane-based regimens for these patients. Recent clinical experience
with irinotecan (Campostar), both alone and combined with gemcitabine, has been
encouraging and relatively well tolerated as second-line therapy.
Based upon these observations, we evaluated the feasibility and efficacy of
first-line treatment with paclitaxel/carboplatin/etoposide followed by
gemcitabine/irinotecan in patients with carcinoma of unknown primary site. We
report here the results of this multicenter phase II study, conducted in the
MPCRN. |