|
|
Male breast cancer
There are a number of good sites that discuss breast cancer in men. There is an NCI site for doctors and the public, some other sites: here, here, here and here. See pictures of MRI or PET scans for male breast cancer here and here
|
INTRODUCTION — Male breast cancer (MBC) is a rare disease, in contrast to female breast cancer, which is the most common cancer and the second leading cause of cancer deaths in women. Although MBC shares many similarities with cancer of the female breast, there are also important differences].
EPIDEMIOLOGY — In the United States, approximately 1690 new cases of MBC will be diagnosed in the year 2005, and 460 deaths will occur; this represents 0.2 percent of all cancers, and approximately 1 percent of all cancer deaths in men annually. By contrast, in Tanzania and areas of central Africa, breast cancer accounts for up to 6 percent of cancers in men. In the United States, the ratio of female to male breast cancer is approximately 100:1 in Caucasians, but lower (70:1) in African Americans.
There are other important epidemiologic differences between male and female breast cancer. The median age of onset of MBC is 65 to 67, approximately 10 years older than for breast cancer in women. The incidence of MBC has remained relatively stable over the last forty years, in contrast to the increased incidence of female breast cancer in both the United States and Europe. This may reflect the impact of screening mammography in women and/or a change in certain risk factors that are specific to women. Screening mammography has not been routinely offered to men, even those at high risk (see below), because of the low frequency of this disease.
Risk factors — Several risk factors for MBC have been identified. In one meta-analysis of published case-control studies, the risk of developing breast cancer was increased in men with the following characteristics: never married, Jewish ancestry, previous benign breast disease, gynecomastia, history of testicular or liver pathology, family history of breast cancer, or prior chest wall irradiation. Testicular conditions associated with an increased risk of MBC include orchitis, undescended testes (cryptorchidism) and testicular injury. Among the chronic liver diseases that have been associated with MBC are cirrhosis, alcoholic liver disease, and schistosomiasis.
Hormonal imbalance — Several of these risk factors involve alterations in estrogen or androgen balance (ie, a relative estrogen excess or lack of androgen). As an example, men with liver disease have increased production of androstenedione from the adrenal glands, enhanced aromatization of androstenedione to estrone, and increased conversion of estrone to estradiol. On the other hand, androgens may convey a protective effect on breast tissue by inhibiting cell proliferation. The association of MBC with prolactinoma, a condition often associated with low plasma testosterone levels, is consistent with this hypothesis.
These associations suggest that relative changes in endogenous hormones may play a causative role in MBC. However, abnormalities in peripherally detectable hormone levels have not been detected in affected men. Furthermore, other conditions associated with an increased estrogen-to-testosterone ratio such as obesity, thyroid disease, use of marijuana, and exogenous estrogen use (eg, transsexuals, treatment of prostate cancer) have a less certain relationship to MBC. Gynecomastia, which is most often drug-related is probably not a precursor for MBC, but may be associated with it because of shared hormonal risk factors.
Klinefelter syndrome — The strongest risk factor for developing MBC is Klinefelter syndrome, a rare condition resulting from the inheritance of an additional X chromosome. The risk of MBC in affected men is 50-fold higher than in men with a normal genotype. The Klinefelter syndrome consists of atrophic testes, gynecomastia, high serum concentrations of gonadotropins (follicle-stimulating hormone, luteinizing hormone), and low serum testosterone levels; the net effect is a high ratio of estrogen-to-testosterone.
Genetics — Approximately 15 to 20 percent of men with breast cancer have a family history of breast cancer, compared to only 7 percent of the general male population [7]. This disparity implies that some families carry genetic mutations that increase their risk for both male and female breast cancer.
In women, 5 to 10 percent of breast cancers are related to genetic predisposition. The breast/ovarian cancer genes, BRCA1 and BRCA2, are thought to account for 80 percent of multiple-case breast cancer families with an autosomal dominant pattern of inheritance.
BRCA2 mutations are a more important predisposing factor for breast cancer in men than are BRCA1 mutations . Between 5 and 15 percent of MBCs are associated with BRCA2 mutations, depending on ethnicity and the strength of the family history. Men who inherit germline BRCA2 mutations have an estimated 6 percent lifetime risk of MBC; this represents a 100-fold higher risk than in the general male population. At least one series suggests that MBC in BRCA mutation carriers is associated with aggressive characteristics (ie, high histologic grade, HER-2/neu overexpression).
Mutations in the PTEN tumor suppressor gene, which causes Cowden's syndrome, have also been associated with MBC
HISTOLOGY — Invasive ductal carcinoma is the most common histologic type of MBC, accounting for 85 to 90 percent of cases. Invasive lobular cancer is rare, in contrast to women, in whom it accounts for up to 15 percent of all cases. Although the male breast can be induced to develop true acini and lobules with estrogenic stimulation, the normal unstimulated male breast lacks these structures.
There are also differences in the incidence and clinical characteristics of ductal carcinoma in situ (DCIS). DCIS accounts for a significantly higher proportion of breast cancer in women compared to men (20 to 25 versus 7 percent). DCIS in men tends to occur at a later age, presents more frequently in an intraductal papillary form, and is more often low grade. Paget's disease and inflammatory breast cancer have been described in men
MOLECULAR, GENETIC, AND BIOLOGIC FACTORS — Limited data are available on biologic, molecular and cytogenetic abnormalities in MBC, and their relationship to tumorigenesis or prognosis.
Hormone receptors — In females, 60 to 70 percent of breast cancers are estrogen receptor (ER) or progesterone receptor (PgR) positive. In contrast, up to 91 percent of MBCs express ER, and up to 96 percent express PgR. ER/PgR expression may provide a growth advantage; some data suggest that ER+/PgR+ tumors have higher proliferative activity than ER-/PgR- tumors
Androgen receptor (AR) expression is detected in 34 to 95 percent of MBCs. However, no association between AR expression and other clinicopathologic features or measures of outcome has been reported. AR gene mutations, which are predominantly in the DNA-binding domain in an area responsible for transcriptional control, have been implicated in the development of MBC. However, their contribution to tumorigenesis is not completely clear, and at least two separate series have failed to document AR mutations in tumor material from a total of 128 MBCs.
Oncogenes and tumor suppressor genes — Alterations in oncogenes and tumor suppressor genes have not been well characterized in MBC. In one series of 111 cases, tumor samples were analyzed for expression of the protooncogenes p53 and HER-2/neu (c-erbB-2). HER-2/neu was overexpressed in 29 percent, similar to the rate of overexpression in women with breast cancer. p53 was overexpressed in 21 percent of cases, somewhat lower than in other reports, in which up to 54 percent of samples were p53 positive. Others have shown only a 15 percent rate of Her-2/neu overexpression in a series of 99 MBCs. Abnormalities in these oncogenes may have an impact on prognosis.
Plasminogen activators — Urokinase plasminogen activator (uPA) converts plasminogen to plasmin, which is involved in the degradation of extracellular matrix during tumor cell invasion. Tumor expression of plasminogen activators and their inhibitors, plasminogen activator inhibitor (PAI) types 1 and 2, have been associated with shorter survival in women with breast cancer section on Plasminogen activators). Few data exist regarding the prognostic value of these factors in men. In one report of 40 MBCs, expression of PAI-1, but not uPA or PAI-2, was associated with a significantly worse prognosis
Cytogenetics — Multiple nonrandom non-germline cytogenetic abnormalities have been described in MBC, including loss of the Y chromosome. The pathogenetic role of these changes is uncertain.
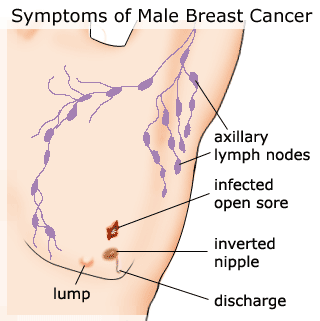
CLINICAL FEATURES — MBC typically presents as a painless, firm mass that is usually subareolar, less often in the upper outer quadrant. The left breast is involved slightly more often than the right, and fewer than 1 percent of cases are bilateral.
Other findings at presentation include nipple retraction, ulceration of the nipple or skin, fixation to skin or underlying muscle, tumor tenderness, and palpable axillary nodes. The reported rate of nipple involvement is 40 to 50 percent, possibly because of the sparsity of breast tissue, and the central location of most tumors. Serosanguinous or bloody nipple discharge is unusual.
DIAGNOSIS AND STAGING — Once a suspicious breast mass is identified in a man, tissue confirmation is mandatory. The differential diagnosis includes gynecomastia, a breast abscess, metastases to the breast, and other non-breast cancer primary tumors (eg, sarcomas). In contrast to MBC, gynecomastia typically presents as a bilateral symmetrical breast enlargement with poorly defined borders, no fixation to the underlying chest wall, and no axillary lymphadenopathy.
Mammography — The mammogram is abnormal in 80 to 90 percent of MBCs, and can usually distinguish between malignancy and gynecomastia. Radiographic features suggestive of malignancy include eccentricity to the nipple, spiculated margins, and microcalcifications. section on Mammographic features of breast cancer). In contrast, gynecomastia typically appears as a round or triangular area of increased density positioned symmetrically in the retroareolar region. In rare cases, concurrent gynecomastia can mask a malignant lesion.
FNA cytology — An adequate tissue sample is important to both establish the diagnosis and to assay for ER and PgR content. Although fine needle aspiration (FNA) cytology can provide adequate diagnostic material in many cases, avoiding open or surgical biopsy up to one-fourth of samples are insufficient for diagnosis. As an example, in one series of 507 men undergoing breast FNA, 23 percent of samples were unsatisfactory, while there were no false positive or false negative cases among those with adequate material. If inadequate tissue is obtained or FNA is not feasible, a core or open biopsy should be performed.
Staging work-up — The diagnostic evaluation and staging for MBC is the same as for women with breast cancer, section on Staging work-up). The staging system developed by the American Joint Committee on Cancer (AJCC) classifies breast malignancies by tumor (T) node (N) and metastasis (M) categories, and stage groupings with similar prognoses are combined . As in women, AJCC stage, tumor size and axillary lymph node status appear to be the most important factors influencing outcome in MBC
TREATMENT OF LOCALIZED DISEASE — Treatment of nonmetastatic MBC follows the same general principles as with female breast cancer. Localized disease is usually treated surgically, with adjuvant treatment considered in most cases.
Surgical resection — The traditional surgical approach for localized MBC is modified radical mastectomy (MRM). Randomized studies have not been conducted in men, but retrospective data suggest the equivalence of radical mastectomy and MRM in terms of local recurrence and survival, and randomized studies in women also support their therapeutic equivalence. The rare man with extensive chest wall muscle involvement may benefit from a radical mastectomy.
Although breast conserving therapy (BCT, lumpectomy followed by breast irradiation) is an appropriate option for many women with early stage disease, it is not usually considered in men because of the lack of breast tissue and the central location of most tumors. As in women, axillary nodal dissection (ALND) is an essential part of surgical therapy.
SLN biopsy — In female breast cancer, sentinel lymph node (SLN) biopsy has emerged as a less morbid alternative to ALND. In experienced hands, the SLN accurately predicts the status of the remaining regional nodes, obviating the need for a complete ALND. SLN biopsy may be successful in men as well. In the larger of these two reports, one or more SLNs were identified in 15 of 16 men with localized disease, using both radiocolloid and blue dye . Six of 10 men with a negative SLN underwent full ALND, and all had negative nodes. Further experience with this technique is needed.
Adjuvant therapy — The low incidence of MBC precludes the development and completion of clinical trials to assess the efficacy of adjuvant therapy, and few prospective data are available to guide therapy. Recommendations for adjuvant endocrine therapy and/or chemotherapy are based largely upon the benefits that have been observed in clinical trials performed in women with early stage breast cancer.
Tamoxifen — Because the majority of MBCs are hormone receptor-positive, five years of adjuvant tamoxifen is frequently recommended following mastectomy, although no prospective randomized controlled trials have confirmed the validity of this approach. Several retrospective comparisons support a benefit from adjuvant tamoxifen in men with localized breast cancer . As an example, in one report that compared 39 men who received tamoxifen to a group of historical controls, the treated group had better five year actuarial survival (61 versus 44 percent) and disease-free survival (56 versus 28 percent).
However, these data may underestimate the degree of benefit of adjuvant tamoxifen, since most men were treated for two years or less, and in women, five years is superior to shorter treatment durations. One possible reason for the shorter treatment duration is that tamoxifen may be tolerated less well by men than by women. In one report of 24 men receiving adjuvant tamoxifen, the most common side effects were:
![]() Decreased libido — 29
percent
Decreased libido — 29
percent
![]() Weight gain — 25 percent
Weight gain — 25 percent
![]() Hot flashes — 21 percent
Hot flashes — 21 percent
![]() Mood alteration — 21
percent
Mood alteration — 21
percent
![]() Depression — 17 percent
Depression — 17 percent
Treatment was discontinued by 21 percent of men because of side effects. This is much greater than the 4 to 7 percent attrition rate previously reported in women receiving adjuvant tamoxifen, although more recent data suggest that up to 50 percent of women become noncompliant by the fourth year of treatment.
Chemotherapy — There is a similar lack of data supporting a benefit for adjuvant chemotherapy in MBC. In one series, eleven men with stage II or III breast cancer were treated with adjuvant chemotherapy (ten with FAC [5-FU, doxorubicin, and cyclophosphamide], and one with CMF [cyclophosphamide, methotrexate, and 5-FU]) after local therapy. Compared to untreated historical controls, adjuvant therapy appeared to favorably influence the risk of recurrence and survival.
In a second report from the National Cancer Institute, 24 men with node-positive, stage II breast cancer were treated with adjuvant CMF. Only 17 of 24 patients were able to complete all 12 planned cycles of therapy, but the favorable five-year survival rate (>80 percent) in this group of patients appears better than historical controls, and suggestive of benefit.
Thus, despite the lack of randomized trials that conclusively demonstrate the benefit of adjuvant chemotherapy in MBC, recommendations for adjuvant systemic chemotherapy that have been adopted for women with early stage disease are generally followed in men at high risk of recurrence. section on Recommendations). The significant variability in adjuvant treatment strategies applied to men and women with breast cancer in the United States were illustrated in a comparative study of 3627 matched pairs of female and male breast cancer cases derived from the National Cancer Data Base, and matched according to the man's age at diagnosis (within five years), ethnicity, income category, and stage. When similar disease categories were studied, men were significantly less likely to receive adjuvant chemotherapy than women (27 versus 41 percent).
RT — No prospective, randomized clinical trials have evaluated the clinical impact of adjuvant RT following MRM. In small retrospective series, postmastectomy RT appears to reduce locoregional recurrence in MBC, but does not improve overall survival. However, RT techniques have varied substantially between series and over time, making an assessment of clinical impact difficult. In women with breast cancer, a survival advantage for postmastectomy irradiation has been shown for patients with node-positive disease in at least two trials., section on Modern clinical trials). Whether these results can be extrapolated to men is unclear, but at least one review from Johns Hopkins suggests that similar indications for postmastectomy RT should be applied to both men and women with breast cancer
Recommendation — For men whose tumors express hormone receptors, five years of adjuvant tamoxifen with or without chemotherapy is recommended. Systemic chemotherapy alone is appropriate for men with hormone receptor-negative disease. Because there are no data with which to determine exactly which men with ER-positive disease will benefit from adjuvant chemotherapy, we use the same guidelines as in women, offering systemic chemotherapy to men with node-positive disease, or tumor size larger than 1 cm. Reasonable choices include an anthracycline-based combinations, or CMF
RT to the chest wall should be considered for men with locally advanced disease or positive axillary nodes, in order to decrease the likelihood of a local recurrence.
TREATMENT OF ADVANCED DISEASE — Due to the high frequency of hormone receptor positivity, men with advanced or metastatic MBC are usually considered initially for hormone therapy, and systemic chemotherapy is reserved for second-line treatment.
Hormone therapy — Effective hormone therapy can be ablative (ie, orchiectomy, adrenalectomy, hypophysectomy) or additive (eg, tamoxifen). The effectiveness of bilateral orchiectomy for advanced MBC was first reported in 1942, subsequent series indicate response rates between 32 and 67 percent However, due to the unwillingness of many men to accept orchiectomy or other surgical forms of therapy (eg, adrenalectomy, hypophysectomy), additive hormone therapy, particularly with tamoxifen, has become the initial treatment of choice for advanced MBC.
As with other hormonal therapies, the objective response rate with tamoxifen is closely linked to hormone receptor expression. Over 80 percent of men with ER-positive advanced disease respond to tamoxifen, but men with ER-negative disease do not benefit. Other endocrine therapies (eg, aminoglutethimide, megestrol acetate, androgens, antiandrogens, steroids, cyproterone acetate, estrogens, luteinizing hormone-releasing hormone analogs) are associated with 50 to 70 percent response rates in ER-positive MBC; side effect profiles tend to be less favorable than with tamoxifen.
Although they are very active in women with hormone receptor-positive advanced breast cancer, selective aromatase inhibitors, such as anastrozole, exemestane, and letrozole, their benefit in men is unclear. At least two case reports suggest benefit, but others recorded no objective responses to anastrozole in five men with ER-positive metastatic disease, three of whom had responded to prior hormonal therapy. However, two patients had stable disease for longer than 6 months.
These alternative endocrine therapies can be considered in men with ER-positive tumors who progress following treatment with tamoxifen, and who do not have rapidly progressive or life-threatening visceral disease. Men responding to one form of hormonal treatment have a greater likelihood of responding to subsequent hormonal manipulations.
Chemotherapy — Chemotherapy should be considered for men with ER-negative tumors, and for those with either rapidly progressing or life-threatening visceral disease. These and other treatment principles are similar to those in women with advanced breast cancer.
The optimal chemotherapy regimen has not been defined in men, although some reports suggest a similar response rate in men as in women with advanced disease. Some anecdotal reports describe the activity of single agent chemotherapy or combination chemotherapy regimens in MBC:
![]() FAC — 67 percent
FAC — 67 percent
![]() Doxorubicin plus vincristine
— 57 percent
Doxorubicin plus vincristine
— 57 percent
![]() Cyclophosphamide alone —
53 percent
Cyclophosphamide alone —
53 percent
![]() CMF — 33 percent
CMF — 33 percent
Other reports are based on fewer than five patients Taxanes have not been systematically investigated; one report of single agent docetaxel included nine men, but the efficacy was not reported separately for men and women
In view of the lack of prospective data, use of the same treatment guidelines as used for women is a reasonable approach.
PROGNOSIS — As in women with breast cancer, tumor size and the presence and number of involved lymph nodes are the most important prognostic factors for MBC. As an example, in two reports involving 335 and 397 cases of MBC, the following 10-year disease-specific survival rates were reported:
![]() Histologically negative nodes
— 77 and 84 percent
Histologically negative nodes
— 77 and 84 percent
![]() One to three positive nodes — 50 and 44 percent
One to three positive nodes — 50 and 44 percent
![]() Four or more histologically positive nodes
— 24 and 14 percent
Four or more histologically positive nodes
— 24 and 14 percent
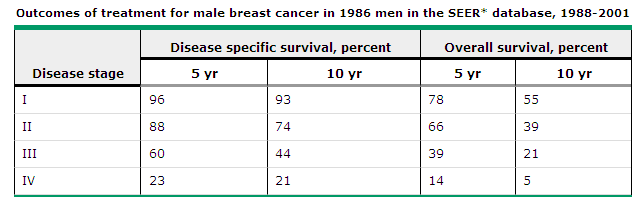
Stratification of outcome according to AJCC tumor stage is illustrated by the following five year disease-free survival rates derived from two different series:
![]() Stage I — 80 and 100
percent
Stage I — 80 and 100
percent
![]() Stage II — 67 and 83
percent
Stage II — 67 and 83
percent
![]() Stage III — 25 and 60
percent
Stage III — 25 and 60
percent
![]() Stage IV — 0 and 25
percent
Stage IV — 0 and 25
percent
Compared to women — In the past, MBC was considered an aggressive disease presenting at a later stage and with a considerably worse prognosis than in women. More recent data have challenged these assumptions. Contemporary reports of both male and female breast cancer that were carefully matched for stage and grade do not substantiate a significantly worse outcome in men.
This absence of gender difference in contemporary series has been attributed by some to improvements in treatment of MBC. However, the data to support this hypothesis are conflicting:
![]() In one report of 217 cases of MBC treated at 18 Wisconsin
institutions between 1953 and 1995, men diagnosed after 1986 tended to present with
earlier stage disease and were more likely to be treated with MRM and adjuvant systemic
therapy. Compared to earlier time periods, these differences coincided with an improved
five year survival.
In one report of 217 cases of MBC treated at 18 Wisconsin
institutions between 1953 and 1995, men diagnosed after 1986 tended to present with
earlier stage disease and were more likely to be treated with MRM and adjuvant systemic
therapy. Compared to earlier time periods, these differences coincided with an improved
five year survival.
![]() Contrasting findings were noted in a report of 229 MBCs presenting
to the Princess Margaret Hospital between 1955 and 1996. Survival and outcome did not
appear to differ over time despite increased use of standard primary surgical treatment
and postoperative chemotherapy and hormone therapy .
Contrasting findings were noted in a report of 229 MBCs presenting
to the Princess Margaret Hospital between 1955 and 1996. Survival and outcome did not
appear to differ over time despite increased use of standard primary surgical treatment
and postoperative chemotherapy and hormone therapy .
Tumor markers — The usefulness of prognostic factors other than stage, tumor size, and nodal status in MBC is uncertain; the disease is rare and few clinical series are large enough or prospectively designed to adequately evaluate specific molecular or pathologic markers. Proposed correlations between prognosis and the expression of various molecular markers such as DNA ploidy, nuclear differentiation, MIB-1 positivity, and cathepsin D have not been confirmed .
A consistent influence of HER-2/neu and p53 expression on prognosis has been noted in most but not all series. In one report, 50 men with invasive ductal carcinoma underwent MRM; 35 also received adjuvant postoperative chemotherapy.The following findings were noted an average follow-up of 59 months:
![]() Men with HER-2/neu-positive tumors had an inferior survival
compared with those who were HER-2/neu-negative (39 versus 96 months).
Men with HER-2/neu-positive tumors had an inferior survival
compared with those who were HER-2/neu-negative (39 versus 96 months).
![]() Men with p53-positive tumors had an inferior survival compared
with those who were p53 negative (33 versus 100 months).
Men with p53-positive tumors had an inferior survival compared
with those who were p53 negative (33 versus 100 months).
![]() All nine patients with tumors that did not express HER-2/neu or
p53 were alive after 58 months compared to none of the 14 with tumors that expressed both
markers.
All nine patients with tumors that did not express HER-2/neu or
p53 were alive after 58 months compared to none of the 14 with tumors that expressed both
markers.
Although these results are impressive, they do not justify routine measurement of either HER-2/neu or p53 in MBC, since there are no data on how to use this information to select specific therapy.
Contralateral breast cancer — The risk of a contralateral breast cancer appears to be higher for men than for women with breast cancer. In one report of 1788 men with breast cancer derived from the SEER database, 12 (0.7 percent) developed a contralateral breast cancer. This represented a marked increase in relative risk compared to men who had not had breast cancer with a standardized incidence ratio (SIR) of 30 overall and 110 in men diagnosed before age 50. The relative risk of contralateral breast cancer in women with breast cancer was much lower (SIR 1.8). However, the absolute risk is much greater in women because of the higher prevalence of the disease.
SUMMARY — A suspicious breast mass in a man must be evaluated by obtaining an adequate tissue sample with FNA or biopsy. If invasive cancer is detected, definitive local therapy is a modified radical mastectomy. As in women, chest wall and regional lymph node irradiation can be considered following mastectomy in men considered to be at high risk of relapse because of axillary lymph node involvement and/or features of locally advanced disease.
Adjuvant systemic treatment recommendation are similar to those for women with early stage breast cancer. Adjuvant therapy should be considered in men with tumor size >1 cm, and for those with axillary nodal metastases. For men whose tumors express steroid hormone receptors, five years of adjuvant tamoxifen with or without chemotherapy should be recommended, while for those with hormone receptor-negative disease, chemotherapy is recommended.
For men with metastatic disease, tamoxifen is the initial treatment of choice for men with hormone-receptor-positive breast cancer. Chemotherapy is recommended for rapidly progressing or life-threatening visceral disease, and for men with hormone receptor-negative disease.