|
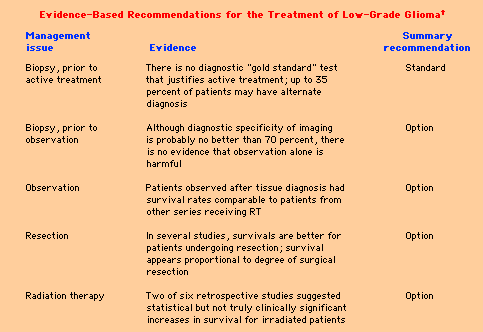
Low-grade glioma Immediate surgical intervention and subsequent radiation therapy (RT) are advocated by some physicians. However, it remains unclear whether early intervention prolongs survival or changes the natural history of the disease, especially in patients who present with a seizure and a normal interictal examination. The classification of gliomas is usually based upon the presumed cell of origin and the degree of malignancy. Classification systems are derived from two original classic schemes: Both of these classification schemes persist in revised form. The first forms the foundation for the WHO classification schema, while the second continues to be used in modified form. LGG is a frequently used term, but it is not clearly defined by either of these classifications. Although a formal definition is not available, LGG may be considered a hybrid term which describes a spectrum of primary brain tumors that are composed of cells which histologically resemble one or more differentiated types of macroglial cells (diffuse and fibrillary astrocytes, oligodendrocytes, ependymal cells) and which have no evidence of anaplasia (in the Kernohan scheme, this would encompass grades I and II). These tumors have also been referred to as "benign" gliomas, but this is a misnomer. Although they have a more favorable prognosis than glioblastomas (GBMs), LGGs are only occasionally associated with prolonged (>10 years) survival and, over their course, frequently develop characteristics similar to more aggressive brain tumors. LGGs occur approximately one-fifth as frequently as the more malignant gliomas. They can develop in any central nervous system (CNS) location, although studies are usually directed to a particular location (ie, LGGs occurring in the cerebral hemispheres, optic pathways, brainstem, etc). Diffuse astrocytoma is the most common histologic subtype of LGG. All diffuse astrocytomas widely infiltrate surrounding neural tissues. They generally occur in adults with a peak age incidence in the late thirties, approximately twenty years younger than GBM. The most common location from which they arise is the cerebral hemispheres; the most common presenting symptom is seizure.Diffuse astrocytomas can be subdivided further into three types: fibrillary, protoplasmic, and gemistocytic. The prognosis for those tumors varies greatly, independent of histology. Ten year survivals can occur in up to 20 percent of patients, while other tumors behave more aggressively with survivals indistinguishable from anaplastic astrocytomas. These distinctions are not absolute. Among gemistocytic tumors, for example, both "pure" and "mixed" subtypes have been noted. The former has been defined as a glial tumor with more than 60 percent gemistocytes and a background of fibrillary astrocytes; the latter has been defined as containing 20 to 60 percent gemistocytes and a background of anaplastic astrocytes. Juvenile pilocytic astrocytomas — Juvenile pilocytic astrocytomas (JPAs) occur almost exclusively in patients less than 25 years of age. They differ microscopically from fibrillary astrocytomas in that there is scant intercellular fibrillary matrix, even though GFAP-positive fibrils are demonstrable in the cell cytoplasm. Tumor cells remote from blood vessels have a rarefied and sparsely cellular appearance and tend to undergo microcystic degeneration. Rosenthal fibers are frequently encountered and are a useful pathologic hallmark in differentiating these tumors from other LGGs.JPAs most frequently arise in the cerebellar hemispheres and around the third ventricle. However, they also are encountered in the cerebral hemispheres. They are frequently cystic and well-demarcated. Although almost always circumscribed on imaging studies, they enhance on CT and MR scanning because their blood vessels undergo chronic glomeruloid degenerative hyalinization. A different mechanism, endothelial proliferation, is responsible for enhancement in anaplastic astrocytomas and GBMs. JPAs are distinguished from other LGGs because of their circumscribed nature, rare progression to GBM, and very favorable prognosis. Malignant transformation of these tumors to higher grades of astrocytoma occurs in less than 5 percent of cases. It may happen more frequently in patients who have been previously irradiated , raising a question of whether this modality should be avoided in these patients. Because it is potentially curable, the goal of surgery should be complete removal. However, multicentric spread may occur, especially with hypothalamic tumors. Low-grade oligodendrogliomas appear as a sheet of rounded cells with a well-defined cytoplasmic membrane and an empty-looking cytoplasm (fried egg appearance) . Their cell density is usually low, but hypercellular areas may be present. A rich thin-walled capillary network, calcification, and cortical invasion with perineuronal, perivascular, or subpial neoplastic cells (ie, satellitosis) are often present.Most oligodendrogliomas are slow-growing, hypodense, sometimes calcified tumors that are usually, but not always, nonenhancing with gadolinium. They constitute approximately 5 percent of intracranial adult gliomas, less if only those tumors with just an oligodendroglioma component are considered (ie, not a mixed tumor, see below). They are more likely to hemorrhage than other low-grade gliomas. Although considered to have a somewhat more favorable prognosis than diffuse astrocytomas, the behavior of oligodendrogliomas is similar, in that younger patients, patients who undergo complete resections, and those with low grade (grade II) tumors, appear to have a better prognosis. Grade III (anaplastic) oligodendrogliomas have a worse outcome. In one series of 100 patients with oligodendroglioma diagnosed over a seven year period, the 2, 5, and 10-year survival rates for those treated for grade II lesions were 88, 88, and 85 percent, respectively. Results were worse for patients with initially low-grade tumors that underwent anaplastic transformation during the follow-up period (2, 5, and 10-year survival 79, 64, and 42 percent, respectively), and the corresponding values for those presenting with de novo grade III tumors were 43, 16, and 15 percent, respectively. Anaplastic oligodendrogliomas and their treatment are considered elsewhere. Gangliogliomas are composed of both neoplastic neurons and astrocytes. They most commonly arise in the temporal lobe and are a frequent fortuitous finding when temporal lobectomies are performed to treat epilepsy. The course is generally benign with prognoses close to that of JPAs; however, these tumors can mimic anaplastic astrocytomas. In one series of 25 patients, for example, the progression-free survival after gross total resection was 78 percent and 63 percent after subtotal resection . There appears to be no role for radiation therapy in the treatment of low-grade gangliogliomas of the temporal lobe. Furthermore, RT may promote malignant degeneration of benign lesions The term mixed glioma is used to refer to LGGs that are composed of admixtures of cells. As an example, tumors composed of distinct areas of diffuse astrocytoma and oligodendroglioma are common. Mixed tumors behave similarly to diffuse astrocytomas with similar median survivals If LGG is defined as a tumor composed of cells that appear histologically similar to CNS glial cells, then a number of less common variants can also be grouped under this category, including: Although uncommonly encountered, they are important to recognize because of their generally good prognosis; further therapy beyond surgery, even when resections are incomplete, is usually not necessary. Ependymomas, tend to occur in young patients and arise in the area of the fourth ventricle. However, they are generally not considered LGGs even though they are composed of cells that have the appearance of ependymal cells, glial cells that line the ventricular cavity. DIAGNOSIS AND CLINICAL COURSE — Although LGG can be defined in terms of pathology, there is often a delay between the time a lesion is identified on imaging studies as a probable LGG and the time that histologic confirmation is obtained. This is because the diagnosis can be made and the clinical course predicted with some degree of certainty without biopsy. LGG is highly likely when a patient presents with a transient neurologic disturbance consistent with seizure, and imaging reveals an unenhancing hemispheric mass that produces little mass effect. Persistent symptoms generally reflect either the location of the tumor (eg, hemiparesis, ataxia) or the result of increased intracranial pressure (eg, headache, change in mental status). These relationships can be illustrated by the following observations: Cranial imaging can be supplemented by the use of positron-emission tomography (PET) scanning. Because LGGs are characterized by glucose hypometabolism, PET images showing diffuse hypometabolism may support a decision to defer treatment, while the presence of hypermetabolic areas may indicated the presence of high-grade tumors, and signal the need for biopsy or treatment. Since one can predict the subsequent clinical course with fair reliability from these parameters alone (ie, isolated transient symptoms and nonenhancement), physicians often delay making a histologic diagnosis until symptoms or imaging worsen. Whether such a delay in diagnosis and treatment significantly affects outcome is controversial and is still the subject of sometimes heated debate. In order to decide whether delaying treatment is detrimental, it would be useful to have controlled studies in which to assess outcome. Unfortunately, studies which address immediate versus delayed surgery and radiation therapy (RT, which is the conventionally used adjuvant therapy) are not available. However, several retrospective studies have addressed outcome after histologic diagnosis is made. Not surprisingly, it is difficult to arrive at a consensus based upon retrospective data. Nevertheless, several general observations can be made from these reports concerning prognosis and progression. JPAs have a much better prognosis than other LGGs. The typical clinical characteristics of these tumors: age less than 25 and well demarcated with contrast enhancement, are so different from the other LGGs that there is sufficient evidence to distinguish them as a separate entity. Independent of their location, the prognosis for patients with JPAs is excellent, even with incomplete tumor resection. RT has resulted in little impact on survival or prevention of tumor progression in JPAsThe generally favorable prognosis in JPA can be illustrated by the following observations. In one series of 34 patients treated by surgical removal of the tumor, 82 percent had a good outcome at a mean follow-up of 17 years. In another report of 51 patients with a median follow-up of 15 years, 82 percent were alive at 10 years; 89 percent of these patients were fully active. Ten-year survival was higher in patients who underwent gross total or radical subtotal tumor resection (100 versus 74 percent in patients with subtotal tumor resection or biopsy alone. Although certain less common LGGs such as gangliogliomas (see above) and pleomorphic xanthoastrocytomas might also have a very favorable prognosis, the prognosis of the other pathologic subtypes of LGGs is similar. Among the more common LGGs, most patients eventually deteriorate. In one series of 53 adults with supratentorial astrocytomas, the majority of whom had either subtotal or gross total resection and postoperative RT, the median survival was 7.3 years with a five-year survival of 64 percent. Deaths were generally due to evolution into an anaplastic astrocytoma or GBM. The median time to recurrence was 4.5 years after the original surgery, and the survival from recurrence was only 12 months. In this and other studies, young age, good performance status, and the clinical presentation of an isolated seizure with an otherwise normal examination were generally good prognostic features. On the other hand, CT contrast enhancement of the original tumor appears to predict a high likelihood of progression to a malignant lesion Mixed tumors have a similar prognosis to diffuse astrocytomas. In one series of 71 patients, for example, the five, ten, and fifteen year survival rates were 55, 29, and 17 percent, respectively; these values were significantly lower than age and sex matched controls. All patients were treated with surgery and 66 with postoperative RT. Tumor grade, using the Kernohan method, was the major prognostic feature. However, even patients with grade 1 and 2 tumors had a median survival of only 6.3 years. Progression was typically associated with anaplastic transformation. Other studies have noted lower survival rates although pathologic grade was still important. More recent data suggests that patients with oligodendroglioma or mixed glioma have a prolonged natural history. In a retrospective review of 106 patients with low-grade oligodendroglioma or mixed glioma, the majority of whom had either biopsy alone or subtotal resection, the median time to progression was five years (range 0.5 to 14.2), and the median overall survival was 17 years. Some of this long survival may reflect the effect of lead time bias since the average time from symptoms to diagnosis was less than one month, which is shorter than in most other series. Tumor progression eventually developed in 68 percent of patients. Disease-free and overall survival did not differ with immediate or deferred therapy (see below). The extent of surgical resection favorably affects outcome. However, excellent outcomes have been described in patients who just underwent biopsy. In one series of 25 such patients, the median survival was 8.2 years. None of the patients died from progressive low-grade disease; seven died as a consequence of their tumors dedifferentiating into a more malignant astrocytoma or GBM, with a median time of approximately five years after the diagnosis.Similar findings have been noted in other studies, suggesting that one could expect a period, usually in the range of five to seven years after diagnosis, of radiographic and clinical stability after which tumors take on characteristics indistinguishable from more anaplastic astrocytomas and glioblastomas. For patients with diffuse astrocytomas or oligodendrogliomas, progression will occur in more than 50 percent of cases. The goals of treatment are to minimize morbidity, prevent tumor enlargement, and prevent transformation of LGG into a more malignant glioma. Symptomatic management usually consists of controlling hydrocephalus resulting from blockage of the CSF drainage pathways, and the treatment of seizures.The seizures associated with hemispherically located LGGs can be a source of major morbidity and are more refractory to medical management than idiopathic epilepsy. In these instances, consideration should be given to performing surgery. Over one-half of patients with a short preoperative duration of seizures (less than one year) will have near complete or total resolution of seizures after tumor resection. Although it is difficult to definitively demonstrate that it is superior to a standard tumor resection, most clinicians advocate seizure-type surgery with intraoperative recording to localize the seizure focus in these instances. Specific antitumor treatments aimed at either preventing or postponing tumor enlargement or malignant transformation include surgery, RT, and chemotherapy. As noted above, several studies suggest that the more extensive the tumor resection, the better the prognosisHowever, specific conclusions cannot be drawn from these series as they all suffer from design-related limitations, particularly small size and the inclusion of histologic tumor types with varying natural histories. Furthermore, it is difficult to distinguish from these retrospective studies whether a polar tumor that presents as an isolated seizure or as a completely resectable lesion has a better prognosis irrespective of what treatments are offered. It therefore remains unclear whether attempting complete resection affords a better prognosis than a stereotactic biopsy, although it may be more effective at controlling morbidity. In the absence of controlled prospective trials, there are several approaches that have been taken. The aggressive approach recommends extensive resection at the time of diagnosis, regardless of symptoms. This approach is based upon the opinion that complete resection may improve survival, and decrease the rate of malignant transformation. Intraoperative magnetic resonance imaging (MRI) has been used to help the surgeon identify small lesions and to improve the extent of tumor resection . Subsequent to surgery, all patients with diffuse astrocytomas, oligodendrogliomas, or mixed tumors undergo RT with dosages to the local tumor bed exceeding 50 Gy. Others, citing the lack of evidence that early intervention is beneficial, prefer to delay aggressive treatment until there is radiographic evidence of tumor growth, intractable seizures, progressive neurologic impairment, or malignant transformation . At least three randomized controlled trials have addressed the impact of RT on low grade astrocytomas. In one trial conducted by the European Organization for Research and Treatment of Cancer (EORTC), 311 patients with supratentorial LGG were randomly assigned following biopsy or subtotal excision to receive either RT (54 Gy in six weeks) or no therapy until progression. Gross total resection was accomplished in 62 percent of patients, and the remainder had biopsy only. With a median follow-up of five years, postoperative RT significantly prolonged progression-free survival (44 versus 37 percent), but had no impact on overall survival (63 versus 66 percent). Two other randomized studies have failed to show a survival benefit from higher RT doses in patients with LGG In phase II studies, hyperfractionated radiation therapy is feasible for incompletely resected lesions with mild to moderate toxicity; however, improved survivals have not been observed. Postoperative fractionated stereotactic radiotherapy also offers an alternative to conventional RT, permitting sparing of irradiation to normal brain tissue. Most authorities agree that radiation therapy is not indicated in the initial management of juvenile pilocytic astrocytoma. In other LGGs, postoperative RT has been recommended after complete resection by some, whereas others advise that it be withheld until there is evidence of tumor recurrence. Although relatively well tolerated, RT is not without potential long term morbidities. As an example, a subacutely progressive radiation necrosis, due to a radiation-induced vasculopathy, can occur 12 to 18 months after therapy is completedAnother potential side effect is neuropsychologic symptoms including cognitive decline. It has been suggested that this complication is overrated, and that cognitive decline is a function not only of RT, but also the tumor itself and antiepileptic drug use. This issue was addressed in several studies: RT itself may promote malignant transformation. Although early studies suggested that this possibility was remote, recent reports suggest a higher incidence of this development in patients with JPA who receive RT Chemotherapy has been occasionally used in LGG with variable results. One controlled trial in patients with incompletely resected LGGs that compared CCNU and radiotherapy to radiotherapy alone failed to show a benefit for chemotherapy in either response rate or median survival. Assessing benefit from chemotherapy can be difficult to judge using standard response criteria, since LGGs are typically nonenhancing, ill-defined, and difficult to measure. Unequivocal clinical improvement that is unaccompanied by a significant reduction in tumor size may indicate benefit, while in some studies, functional imaging (eg, with thallium-201 SPECT) appears to be a better predictor of tumor control and patient survival than CT or MRI. Nevertheless, the end points of most clinical trials are objective response rate and time to tumor progression. LGGs with an oligodendroglioma component may respond to various chemotherapies, particularly the PCV regimen of procarbazine, CCNU (lomustine), and vincristine . Anaplastic (grade III) oligodendrogliomas tend to have a higher response rate, but grade II oligodendroglial tumors are also sensitive.In one study, for example, 52 patients with oligodendroglioma or mixed oligoastrocytoma were treated with up to six cycles of PCV Thirty-three patients responded, nine completely (objective response rate 63 percent). Response rates were higher in patients with necrosis in the histologic specimen (76 versus 45 percent). The median time to progression was 25 months in complete responders, 12 months in partial responders, and 7 months in nonresponders. A somewhat lower response rate was noted in a second series, in which 28 patients with residual tumor on MRI scan following either biopsy or subtotal resection of low grade oligodendroglioma or oligoastrocytoma received up to six cycles of modified PCV (procarbazine 75 mg/m2 orally days 8 to 21, lomustine 130 mg/m2 orally on day 1, vincristine 1.4 mg/m2 days 8 and 29, every eight weeks) followed by RT within 10 weeks, or immediately in the absence of a chemotherapy response. Eight (29 percent) had objective tumor regression prior to RT. PCV chemotherapy may provide a reasonable alternative to radiation for the initial treatment of large oligodendrogliomas; in such cases, deferred radiation may postpone or avoid the development of delayed leukoencephalopathy. However, randomized trials will be needed to confirm the efficacy of this approach. LGGs are also responsive to second-line as well as first-line temozolomide Both grade II and grade III (anaplastic) oligodendrogliomas and oligoastrocytomas that harbor allelic loss of chromosome 1p or combined allelic losses of 1p and 19q are associated with increased sensitivity to chemotherapy, and longer recurrence-free survival and overall survival. These deletions can be detected by fluorescent in situ hybridization (FISH) analysis of paraffin-embedded tissues. Recommended approach to the patient — Extensive data are not available to guide decision making in the management of patients with LGGs. One group analyzed all the available data on LGGs in an attempt to develop a practice parameter for adults with suspected or known supratentorial nonoptic pathway low-grade gliomas. A search of the world's literature uncovered 59 citations which were deemed acceptable (original work published in peer-reviewed journals) directly or indirectly addressing the natural history, survival, tumor recurrence or quality of life of adult patients managed with observation, biopsy, resection or radiation therapy) for review. The following issues were addressed: The results of this study are summarized in table. The only issue in which there was felt to be consensus was that there was no test except histologic verification that could ensure the diagnosis of LGG to a sufficient degree to justify radiation or chemotherapy. As for the other issues, some evidence was available that suggested a benefit of both extensive surgery and radiation, but no practice parameters could be defined based upon the available evidence and any recommendations could only be considered as optional. In particular, observation of a patient who presents with a seizure and imaging studies consistent with LGG could not be considered substandard care at this time.
|