Semin Radiat Oncol 2000
Oct;10(4):324-32
Neoadjuvant therapy and
surgical resection for locally advanced non-small cell lung cancer.
Meko J, Rusch VW Thoracic Service, Department
of Surgery, Memorial Sloan-Kettering Cancer Center, New York, NY 10021, USA.
During the past 15 years, treatment of stage IIIA
(N2) non-small cell lung cancer has evolved considerably because of improvements in
patients selection, staging, and combined modality therapy. Results
of several clinical trials suggest that induction chemotherapy or chemoradiation and
surgical resection is superior to surgery alone. However, the optimal induction regimen
has not been defined. An intergroup trial is also underway to determine whether
chemoradiation and surgical resection leads to better survival than chemotherapy and
radiation alone.
Ugeskr Laeger 1999 Nov 8;161(45):6169-73
Neoadjuvant chemotherapy in non-small-cell lung
cancer
Larsen SS, Pedersen JJ, Krasnik M, Jensen TS
Over the past 10 years, several studies of
neoadjuvant chemotherapy for stage III Non Small Cell Lung Cancer (NSCLC) have been
conducted. In eight Phase II studies, response rates of
approximately 65% and resectability rates of approximately 50% have been achieved,
with acceptable side effects. Two randomized trials with a total of
120 patients with stage IIIA cancer have been carried out to compare the effect of
neoadjuvant chemotherapy plus surgery versus surgery alone. The trials were terminated
early when interim analysis showed significantly increased survival in groups that
received neoadjuvant chemotherapy. The incidences of treatment-related death did
not differ significantly between the groups. Neoadjuvant chemotherapy for patients in
stage III NSCLC is feasible and might prolong survival. However, the results of larger
randomized trials must be awaited before firm general recommendations can be made.
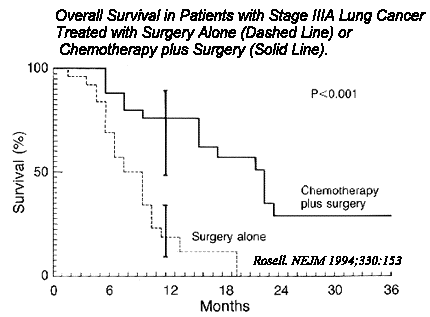
N Engl J Med 1994 Jan 20;330(3):153-8
A randomized trial comparing preoperative
chemotherapy plus surgery with surgery alone in patients with non-small-cell lung cancer.
Rosell R, Gomez-Codina J, Camps C, Maestre J,
Padille J, Canto A, Mate JL, Li S, Roig J, Olazabal A, et al Department of Medical
Oncology, University of Barcelona, Hospital de Badalona Germans Trias i Pujol, Spain.
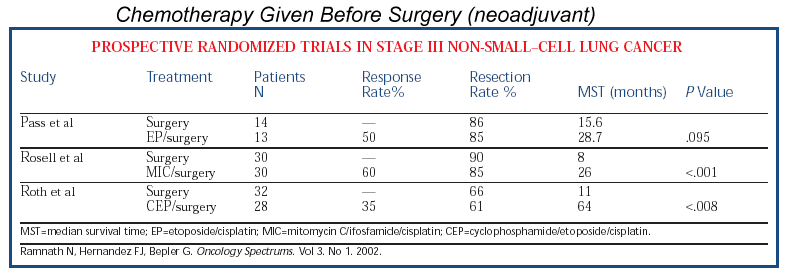
We studied 60 patients (59 men and 1 woman) with stage IIIA non-small-cell lung cancer. The patients were randomly
assigned to receive either surgery alone or three courses of chemotherapy (6 mg of
mitomycin per square meter of body-surface area, 3 g of ifosfamide per square meter, and
50 mg of cisplatin per square meter) given intravenously at three-week intervals and
followed by surgery. All patients received mediastinal radiation after surgery. The
resected tumors were evaluated by means of K-ras oncogene analysis and flow cytometry.
RESULTS. The median period of survival was 26 months in the
patients treated with chemotherapy plus surgery, as compared with 8 months in the patients
treated with surgery alone (P < 0.001); the median period of disease-free survival was
20 months in the former group, as compared with 5 months in the latter (P <
0.001). The rate of recurrence was 56 percent in the group treated with chemotherapy plus
surgery and 74 percent in the group treated with surgery alone. The prevalence of mutated
K-ras oncogenes was 15 percent among the patients receiving preoperative chemotherapy and
42 percent among those treated with surgery alone (P = 0.05). Most of the patients treated
with chemotherapy plus surgery had tumors that consisted of diploid cells, whereas the
patients treated with surgery alone had tumors with aneuploid cells. CONCLUSIONS.
Preoperative chemotherapy increases the median survival in patients with non-small-cell
lung cancer.
J Natl Cancer Inst 1994 May
4;86(9):673-80
A randomized trial comparing perioperative
chemotherapy and surgery with surgery alone in resectable stage IIIA non-small-cell lung
cancer.
Roth JA, Fossella F, Komaki R, Ryan MB,
Putnam JB Jr, Lee JS, Dhingra H, De Caro L, Chasen M, McGavran M, et al Department of
Thoracic and Cardiovascular Surgery, University of Texas M. D. Anderson Cancer Center,
Houston 77030.
BACKGROUND: Patients with resectable stage IIIA non-small-cell lung cancer have a low survival rate following
standard surgical treatment. Nonrandomized trials in which induction chemotherapy or a
combination of chemotherapy and radiation prior to surgery were used to treat patients
with regionally advanced primary cancers have suggested that survival is improved when
compared with treatment by surgery alone. PURPOSE: We performed a prospective, randomized
study of patients with previously untreated, potentially resectable clinical stage IIIA
non-small-cell lung cancer to compare the results of perioperative chemotherapy and
surgery with those of surgery alone. METHODS: This trial was designed to test the null
hypothesis that the proportion of patients surviving 3 years is 12% for either treatment
group against the alternate hypothesis that the 3-year survival rate would be 12% in the
surgery alone group and 32% in the perioperative chemotherapy group. The estimated
required sample size was 65 patients in each group. The trial was terminated at an early
time according to the method of O'Brien and Fleming following a single unplanned interim
analysis. The decision to terminate the trial was based on ethical considerations, the
magnitude of the treatment effect, and the high degree of statistical significance
attained. In total, 60 patients were randomly assigned between 1987 and 1993 to receive
either six cycles of perioperative chemotherapy (cyclophosphamide,
etoposide, and cisplatin) and surgery (28 patients) or surgery alone (32 patients).
For patients in the former group, tumor measurements were made before each course of
chemotherapy and the clinical tumor response was evaluated after three cycles of
chemotherapy; they then underwent surgical resection. Patients who had documented tumor
regression after preoperative chemotherapy received three additional cycles of
chemotherapy after surgery. RESULTS: After three cycles of preoperative chemotherapy, the
rate of clinical major response was 35%. Patients treated with perioperative chemotherapy
and surgery had an estimated median survival of 64 months compared with 11 months for
patients who had surgery alone (P < .008 by log-rank test; P < .018 by Wilcoxon
test). The estimated 2- and 3-year survival rates were 60% and
56% for the perioperative chemotherapy patients and 25% and 15% for those who had surgery
alone, respectively. CONCLUSIONS: In this trial, the treatment strategy using
perioperative chemotherapy and surgery was more effective than surgery alone.
IMPLICATIONS: This clinical trial strengthens the validity of using perioperative
chemotherapy in the management of patients with resectable stage IIIA non-small-cell lung
cancer. Further investigation of the perioperative chemotherapy strategy in earlier stage
lung cancer is warranted.
Lung Cancer 1998 Jul;21(1):1-6
Long-term follow-up of patients enrolled in a
randomized trial comparing perioperative chemotherapy and surgery with surgery alone in
resectable stage IIIA non-small-cell lung cancer.
Roth JA, Atkinson EN, Fossella F, Komaki R,
Bernadette Ryan M, Putnam JB Jr, Lee JS, Dhingra H, De Caro L, Chasen M, Hong WK
Our previously reported randomized study of
patients with untreated, potentially resectable clinical stage IIIA non-small-cell lung
cancer found that patients treated with perioperative chemotherapy and surgery had a
significant increase in median survival compared to patients treated with surgery alone.
We have now re-analyzed the results of the study with a median
time from random allocation to analysis for all patients of 82 months. The increase in
survival conferred by perioperative chemotherapy was maintained during the period of
extended observation.
J Clin Oncol 1995 Aug;13(8):1880-92
Concurrent cisplatin/etoposide plus chest
radiotherapy followed by surgery for stages IIIA (N2) and IIIB non-small-cell lung cancer:
mature results of Southwest Oncology Group phase II study 8805.
Albain KS, Rusch VW, Crowley JJ, Rice TW,
Turrisi AT 3rd, Weick JK, Lonchyna VA, Presant CA, McKenna RJ, Gandara DR, et al Loyola
University Medical Center, Maywood, IL, USA.
PURPOSE: To assess the feasibility of concurrent
chemotherapy and irradiation (chemoRT) followed by surgery in locally advanced
non-small-cell lung cancer (NSCLC) in a cooperative group setting, and to estimate
response, resection rates, relapse patterns, and survival for stage subsets IIIA(N2)
versus IIIB. PATIENTS AND METHODS: Biopsy proof of either positive N2 nodes (IIIAN2) or of
N3 nodes or T4 primary lesions (IIIB) was required. Induction was
two cycles of cisplatin and etoposide plus concurrent chest RT to 45 Gy. Resection
was attempted if response or stable disease occurred. A chemoRT boost was given if either
unresectable disease or positive margins or nodes was found. RESULTS: The median follow-up
time for 126 eligible patients [75 stage IIIA(N2) and 51 IIIB] was 2.4 years. The
objective response rate to induction was 59%, and 29% were stable. Resectability was 85%
for the IIIA(N2) group eligible for surgery and 80% for the IIIB group. Reversible grade 4
toxicity occurred in 13% of patients. There were 13 treatment-related deaths (10%) and 19
others (15%) died of causes not related to toxicity or tumor. Of 65 relapses, 11% were
only locoregional and 61% were only distant. There were 26 brain relapses, of which 19
were the sole site or cause of death. There was no survival
difference (P = .81) between stage IIIA(N2) versus stage IIIB (median survivals, 13 and 17
months; 2-year survival rates, 37% and 39%; 3-year survival rates, 27% and 24%).
The strongest predictor of long-term survival after thoracotomy was absence of tumor in
the mediastinal nodes at surgery (median survivals, 30 v 10 months; 3-year survival rates,
44% v 18%; P = .0005). CONCLUSION: This trimodality approach was feasible in this
Southwest Oncology Group (SWOG) study, with an encouraging 26% 3-year survival rate. An
Intergroup study is currently being conducted to determine whether surgery adds more to
the risk or to the benefit of chemoRT.
J Thorac Cardiovasc Surg 1995
Mar;109(3):473-83; discussion 483-5
Results of cancer and leukemia group B protocol
8935. A multiinstitutional phase II trimodality trial for stage IIIA (N2) non-small-cell
lung cancer. Cancer and Leukemia Group B Thoracic Surgery Group.
Sugarbaker DJ, Herndon J, Kohman LJ, Krasna
MJ, Green MR Division of Thoracic Surgery, Brigham & Women's Hospital, Harvard Medical
School, Boston, MA 02115.
From October 1989 to February 1992, 74 patients
with mediastinoscopically staged IIIA (N2) non-small-cell
lung cancer from 30 CALGB-affiliated hospitals received two cycles
of preresectional cisplatin and vinblastine chemotherapy. Patients with responsive
or stable disease underwent standardized surgical resection and radical lymphadenectomy.
Patients who underwent resection received sequential adjuvant therapy with two cycles of
cisplatin and vinblastine, followed by thoracic irradiation (54 Gy after complete
resection and 59.4 Gy after incomplete resection or no resection at 1.8 Gy per fraction).
There were no radiographic complete responses to the neoadjuvant chemotherapy, although 65
(88%) patients had either a response or no disease progression. During induction
chemotherapy, disease progressed in seven patients (9%). Sixty-three patients (86%) had
exploratory thoracotomy, and 46 of those (75%) had resectable lesions. A complete surgical
resection was accomplished in 23 patients, and 23 patients had an incomplete resection
with either a diseased margin or diseased highest node resected. Operative mortality was
3.2% (2/63). In 10 patients (22% of the 46 having resection) the disease was
pathologically downstaged. There was no correlation between radiographic response to the
induction chemotherapy and downstaging at surgical resection. The full protocol was
completed by 33 patients (45% of original cohort). Overall
survival at 3 years was 23%. Patients undergoing resection had significantly improved
survival at 3 years compared with patients not having resection: 46% for complete
resection (median 20.9 months), 25% for incomplete resection (median 17.8 months), and 0%
for no resection (median 8.5 months). Five deaths occurred during the treatment
period. A total of 18 of the 46 (39%) patients who underwent resection are either alive
and disease-free or have died without recurrence.
Chest 1994 Dec;106(6 Suppl):348S-354S
Randomized phase 2 evaluation of preoperative
radiation therapy and preoperative chemotherapy with mitomycin, vinblastine, and cisplatin
in patients with technically unresectable stage IIIA and IIIB non-small cell cancer of the
lung. LCSG 881.
Wagner H Jr, Lad T, Piantadosi S, Ruckdeschel
JC Department of Radiation Oncology, Albany Medical College, NY.
Between June 1988 and January 1980, 67 patients
with pathologic stage III non-small cell lung cancer were randomized to receive either preoperative mitomycin, vinblastine, and cisplatin (MVP) chemotherapy
(cisplatin 120 mg/m2, and mitomycin, 8 mg/m2 day 1 + 29, and vinblastine, 4.5 mg/m2 on day
1, 15, 22, and 29 and 2.0 mg/m2 day 8), or preoperative radiotherapy (44 Gy in 22
fractions to the primary tumor and mediastinum). The purpose of this study was to
identify a treatment approach that showed sufficient effectiveness and acceptable toxicity
to warrant testing by prospective randomized trial against "standard"
nonsurgical treatment. All patients had surgical staging of the mediastinum and had either
unresectable N2 disease or T4 disease with proximal extension of disease along the
pulmonary artery. Response to preoperative therapy was evaluated 8 weeks after beginning
treatment and patients with complete or partial radiographic response were to undergo
surgical exploration and resection if possible. Fifty-seven patients were eligible and
evaluable for response. Of the 67 total patients, 3 were unavailable for follow-up, 4 were
ineligible, 1 was canceled, and 2 refused all treatment after having been randomized. Of
the eligible and evaluable patients, 49 had stage IIIA and 8 had stage IIIB disease.
Randomization was to MVP in 26 cases and to radiotherapy (XRT) in 31. Radiographic
response to treatment was virtually identical for the two approaches, with 29 of the 57
evaluable patients achieving objective responses. In patients achieving radiographic
response, 24 underwent surgical exploration and 20 underwent resection, of which 18 were
complete. The mediastinum was free of tumor in seven patients but only two pathologic
complete responses were seen (one each to XRT and MVP). In addition, ten nonresponders
underwent surgery; seven underwent resection. Median survival for the entire group is 12
months, with a 27% actuarial survival at 4 years. Two patients died of treatment toxicity
during preoperative therapy. Overall toxicity included 2 preoperative toxic deaths and 6
postoperative deaths in 34 patients who underwent surgical exploration (3 each with XRT
and MVP) due to adult respiratory distress syndrome (3), myocardial infarction (1),
pulmonary edema (1), and esophageal fistula (1), for an overall death rate 8 of 57 (14%)
and a perioperative death rate in surgically explored patients of 6/34 (18%). These preoperative regimens, in the population studied herein, were of
modest efficacy and substantial toxicity.
J Thorac Cardiovasc Surg 2001
Mar;121(3):472-83
Induction chemoradiation and surgical resection
for non-small cell lung carcinomas of the superior sulcus: Initial results of Southwest
Oncology Group Trial 9416 (Intergroup Trial 0160).
Rusch VW, Giroux DJ, Kraut MJ, Crowley J,
Hazuka M, Johnson D, Goldberg M, Detterbeck F, Shepherd F, Burkes R, Winton T, Deschamps
C, Livingston R, Gandara D Thoracic Service, Department of Surgery, Memorial
Sloan-Kettering Cancer Center, New York, NY, USA.
OBJECTIVE: The rate of complete resection (50%)
and the 5-year survival (30%) for non-small cell lung carcinomas of the superior sulcus
have not changed for 40 years. Recently, combined modality therapy has improved outcome in
other subsets of locally advanced non-small cell lung carcinoma. This trial tested the
feasibility of induction chemoradiation and surgical resection in non-small cell lung
carcinoma of the superior sulcus with the ultimate aim of improving resectability and
survival. METHODS: Patients with mediastinoscopy-negative T3-4 N0-1 superior sulcus
non-small cell lung carcinoma received 2 cycles of cisplatin and
etoposide chemotherapy concurrent with 45 Gy of radiation. Patients with stable or
responding disease underwent thoracotomy 3 to 5 weeks later. All patients received 2 more
cycles of chemotherapy and were followed up by serial radiographs and scans. Survival was
calculated by the Kaplan-Meier method and prognostic factors were assessed for
significance by Cox regression analysis. RESULTS: From April 1995 to September 1999, 111
eligible patients (77 men, 34 women) were entered in the study, including 80 (72.1%) with
T3 and 31 with T4 tumors. Induction therapy was completed as planned in 102 (92%)
patients. There were 3 treatment-related deaths (2.7%). Cytopenia was the main grade 3 to
4 toxicity. Of 95 patients eligible for surgery, 83 underwent thoracotomy, 2 (2.4%) died
postoperatively, and 76 (92%) had a complete resection. Fifty-four (65%) thoracotomy
specimens showed either a pathologic complete response or minimal microscopic disease. The 2-year survival was 55% for all eligible patients and 70% for patients
who had a complete resection. To date, survival is not significantly influenced
by patient sex, T status, or pathologic response. CONCLUSIONS: (1) This combined modality
treatment is feasible in a multi-institutional setting; (2) the pathologic complete
response rates were high; and (3) resectability and overall survival were improved
compared with historical experience, especially for T4 tumors, which usually have a grim
prognosis.
Br J Cancer 1998 Sep;78(5):683-5
Randomized study of chemotherapy and surgery
versus radiotherapy for stage IIIA non-small-cell lung cancer: a National Cancer Institute
of Canada Clinical Trials Group Study.
Shepherd FA, Johnston MR, Payne D, Burkes R,
Deslauriers J, Cormier Y, de Bedoya LD, Ottaway J, James K, Zee B Interdepartmental
Division of Oncology of the University of Toronto, Ontario, Canada.
Thirty-one patients with stage IIIA (N2)
non-small-cell lung cancer were randomized to receive radiotherapy alone or chemotherapy
with cisplatin and vinblastine followed by surgery. Response rates to induction
chemotherapy and radiotherapy were 50% and 53.3% respectively. Complete surgical resection
was possible for 62.5% of patients. Median survival times were
16.2 and 18.7 months for radiotherapy alone and chemotherapy-surgery respectively (P =
Ns), with no long-term improvement in survival seen with combined-modality treatment. |