| We randomly assigned 85
patients from 10 centers to receive either radiotherapy and
supportive care or supportive care alone. The trial was
discontinued at the first interim analysis, which showed that
with a preset boundary of efficacy, radiotherapy and supportive
care were superior to supportive care alone. A final analysis
was carried out for the 81 patients with glioblastoma (median
age, 73 years; range, 70 to 85). At a median follow-up of 21
weeks, the median survival for the
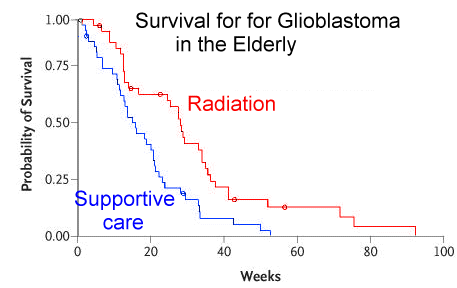
39 patients who received radiotherapy plus supportive care was
29.1 weeks, as compared with 16.9 weeks for the 42 patients
who received supportive care alone. The hazard ratio for
death in the radiotherapy group was 0.47 (95% confidence
interval, 0.29 to 0.76; P=0.002). There were
no severe adverse events
related to radiotherapy. The results of quality-of-life and
cognitive evaluations over time did not differ significantly
between the treatment groups.
Conclusions Radiotherapy results in a
modest improvement in survival, without reducing the quality of
life or cognition, in elderly patients with glioblastoma.
This study shows that the addition of radiotherapy to supportive
care prolongs survival and does not reduce the health-related
quality of life or cognitive function of patients with newly
diagnosed glioblastoma who are 70 years of age or older.
The
16.9-week median
survival of patients in our study who received
only supportive care
is consistent with the median survival
reported more than
two decades ago for younger patients treated
with supportive care
alone. Conversely, the
12.2-week survival
benefit with
radiotherapy in the older patients in our study
is about half the
survival gain reported in the two earlier
studies (22 and 24
weeks), which compared conventional radiotherapy
(a total dose of 45
to 60 Gy, given in fractions of 1.7 to 2.0
Gy) with supportive
care in a younger population
We selected a conventional 50-Gy schedule
to minimize the age-related risk of radiation-induced
neurotoxicity. With this schedule, there were no cases of
delayed neurotoxicity,but the short survival of the patients in
our study may have precluded the development of late toxicity.
The optimal dose of radiotherapy in elderly patients remains
undetermined. It is unclear whether a total dose of 60 Gy would
increase the survival benefit of radiotherapy in older
patients, as it does in younger patients. In our trial, the
29.1-week median survival and 15% rate of discontinuation of
radiotherapy compare favorably with the 22.1-week survival and
26% rate of discontinuation reported in a prospective study in
which a dose of 60 Gy was delivered in 30 fractions over a
period of 6 weeks in older patients. In that study, an
abbreviated course of radiotherapy (40 Gy in 15 fractions over
a period of 3 weeks), as compared with the 60-Gy schedule,
resulted in a similar median
survival (24.3 weeks vs. 22.1 weeks), but a lower rate
of premature discontinuation of radiotherapy (10% vs. 26%).
Since the goal of the treatment of
glioblastoma in older patients is palliation, the quality of
life is relevant. The evaluation of health-related quality of
life in patients with malignant glioma is notoriously
difficult.The main limitation is that severely ill patients
with a rapidly progressive, fatal disease are not always
compliant with such evaluations. To our knowledge, only one
prospective study in the elderly attempted to evaluate
health-related quality of life sequentially in patients with
glioblastoma, but the data could not be analyzed because the
compliance rate was too low (15 to 21% immediately after
radiotherapy).17
In our study, the rate of compliance with the questionnaires
was similar to that in the few reported studies of patients
with glioblastoma. We were able to conduct a meaningful
analysis of the data obtained from the first four follow-up
evaluations (135 days of follow-up). Thereafter, the number of
patients was too small for a reliable analysis.
At baseline, scores for the health-related
quality of life did not differ significantly between the two
groups and were similar to scores reported previously.During
and after treatment, scores on several evaluation scales
(physical, cognitive, social, fatigue, and motor dysfunction)
progressively declined in both groups, although the global
score for health-related quality of life did not change
significantly over time in either group. We did not observe the
mild-to-moderate improvement on several scales that was
reported in younger patients after radiotherapy, with or
without chemotherapy.
The evaluation of neuropsychiatric symptoms
and cognitive function is associated with the same compliance
limitation as the health-related quality-of-life analysis. The
Neuropsychiatric Inventory did not show a time or treatment
effect, particularly in the case of depression. In contrast,
the cognitive evaluation (the MMSE and the initiation subscale
of the MDRS) showed significant deterioration over time in both
groups. However, as compared with supportive care alone,
radiotherapy plus supportive care did not have a detrimental
effect on cognitive function.
The population of elderly patients with
cancer is underrepresented in clinical trials. Likely causes of
this underrepresentation are study-imposed restrictions,
coexisting conditions, concern about the toxic effects of
treatment, patient and family preferences, and the reluctance
of physicians to enroll elderly patients in clinical trials.
Nevertheless, our study shows that these barriers may be
overcome, even in trials that involve a rapidly progressive,
fatal disease, such as glioblastoma, and a palliative-care
comparison group.
In conclusion, radiotherapy increases the
median survival of elderly patients with glioblastoma who have
a good performance status at the start of treatment. As
compared with supportive care, radiotherapy in such patients
does not cause further deterioration in the Karnofsky
performance status, health-related quality of life, or
cognitive functions, but the survival benefit is modest. |