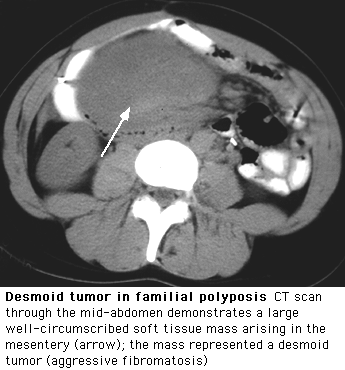
| Desmoid tumors are generally classified as intraabdominal
or extraabdominal. The extraabdominal tumors, which are usually sporadic,
are effectively treated with local therapy. Despite their benign
character, desmoid tumors have a high rate of recurrence with surgery
alone. Patients with multiple local recurrences despite adequate local
therapy are considered for systemic therapy. In contrast, intraabdominal
desmoids, particularly those arising in the setting of FAP, are often
unresectable because they are characterized by diffuse infiltration of the
mesentery. In addition, recurrences tend to become more frequent and
aggressive with each surgical intervention. Systemic therapy options for
these patients include antiinflammatory agents, hormonal agents (eg,
tamoxifen), and cytotoxic chemotherapy. Thus, desmoid tumors
(particularly when they arise in FAP) defy at least two general dogmas
that are prevalent in clinical oncology: that low-grade tumors with no
known metastatic potential do not kill patients, and that they should not
respond to chemotherapy.
EPIDEMIOLOGY —
Desmoid tumors are uncommon; they account for about 0.03 percent of all
neoplasms, and less than 3 percent of all soft tissue tumors. The
estimated incidence in the general population is two to four per million
population per year, which in the United States, translates into
approximately 900 new tumors annually
Individuals between the ages of 15 and 60 are most often affected;
desmoids are rare in the young and in the elderly. They are slightly more
common in women than in men and there is no significant racial or
ethnic distribution.
The incidence of desmoids is higher in patients with FAP. Desmoids
affect from 4 to 20 percent of patients with FAP . The simultaneous
appearance of FAP and desmoid tumors was described by Gardner in 1951, and
is now designated as Gardner's syndrome Until early elective
colectomy became routine in patients with FAP, the dominant cause of death
in these patients was carcinoma of the colon. With the increasing use of
prophylactic colectomy, desmoid tumors have become an important cause of
morbidity and in some instances, mortality Desmoids are responsible
for death in up to 11 percent of patients with FAP
HISTOLOGY —
Desmoids tend to be large bulky tumors that lack pseudoencapsulation, and
locally infiltrate adjacent tissue structures. Histologically, they are
characterized by small bundles of spindle cells in an abundant fibrous
stroma. The fibroblasts have a propensity to concentrate at the periphery
of the lesion, and the cellularity is low. There are usually few mitotic
figures and necrosis is absent.
ETIOLOGY AND PATHOGENESIS
— The etiology of desmoid tumors is unknown. However, the identification
of clonal chromosomal changes in a significant fraction of cases
supports the
neoplastic
nature of these tumors and emerging evidence implicates
dysregulated wound healing in the pathogenesis of these and other
fibroblastic lesions.
Trisomy 8 and 20
— Nonrandom clonal chromosomal changes, particularly trisomy 8 and/or 20,
occur in one-third or more of sporadic desmoid tumors Similar
nonrandom genetic aberrations have been found in benign fibrous bone
lesions (such as fibrous dysplasia), suggesting a similar pathogenesis
Although the clinical relevance of these genetic abnormalities is unclear,
their presence appears to be associated with a higher risk of recurrence
As an example, in one report, trisomy 8 and/or trisomy 20 were observed
in cells cultured from 6 of 13 desmoid tumors FISH (fluorescence in
situ hybridization) analysis performed on the nuclei from 25 desmoid
tumors from paraffin blocks or frozen tissue indicated that local
recurrence was more likely in desmoids with trisomy. Among patients
followed for more than one year, local recurrence was more frequent in
tumors with trisomy 8 (four of six as compared to 2 of 17 recurrences in
trisomy 8-negative tumors).
The finding of individual trisomies and their association in the same
cell is rare in solid tumors, particularly mesenchymal tumors
However, these aberrations are known to occur in related benign, fibrous
lesions arising in both soft tissue and bone tumors, (eg, Dupuytren's
contracture, plantar fibrosis, Peyronie's disease, carpal tunnel syndrome
and infantile fibrosarcoma) Trisomy 8 is also a frequent finding in
hematologic malignancies, but is remarkably infrequent in nonfibrous solid
tumors.
Gardner's syndrome
— Gardner's syndrome is a variant of FAP that is distinguished by the
presence of prominent extraintestinal lesions, such as desmoid tumors,
osteomas, and cysts. When in any member of an FAP family, the family has
traditionally been said to have Gardner's syndrome rather than FAP, since
all members of the family segregate the same mutation in the adenomatous
polyposis coli (APC) gene. Desmoids may be the first manifestation of
Gardner's syndrome. Families have also been reported that exhibit desmoids
as the only manifestation of an APC mutation.
The estimated risk of developing a desmoid tumor in patients with FAP
is between 4 and 20 percent They have a particular predilection for
surgical sites (eg, the mesentery or abdominal wall following
colectomy, the site of an ileal pouch-anal anastomosis).
In one series, prior abdominal surgery had been performed in 68 percent
of patients with FAP and abdominal desmoid tumors; lesions develop within
five years after surgery in approximately one-half [23].
One patient dramatically demonstrated this association with surgery. She
had been treated, apparently successfully with chemotherapy for an
intraabdominal desmoid tumor. To confirm the response, laparoscopy was
performed. Within months, desmoid tumors began developing in each of the
three trocar sites; the tumors became massive and inoperable and led to
the death of the patient.
APC mutations and beta-catenin
— Mutations of the APC gene on chromosome 5q are responsible for FAP. More
than 300 mutations have been described, most of which lead to frame shifts
or premature stop codons, resulting in a truncated APC gene product.
Several studies have attempted to correlate specific APC mutations with
the clinical phenotype. As a general rule, mutations between codons 169 to
1393 are associated with the classic form of FAP while mutations that are
more 3' or 5' are associated with the attenuated form of FAP.
The site of the germline mutation in patients with FAP may be important
for the risk of developing a desmoid tumor. Mutations between codons 1445
and 1578 have been associated with desmoid tumors in some reports
although others have not found this association
How the abnormal gene promotes the formation of tumors such as desmoids
is incompletely understood. However, increasing evidence points to
involvement of germline APC mutations in the molecular pathogenesis of
desmoids in patients with Gardner's syndrome The normal APC protein
prevents the accumulation of beta-catenin, a cytosolic and nuclear
protein, by mediating its phosphorylation and resultant degradation. The
loss of the beta-catenin regulatory domain in the truncated protein allows
beta catenin to accumulate, bind to and activate the transcription factor
Tcf-4
Trauma and pregnancy
— Up to 30 percent of patients with desmoid tumors have a history that
involves antecedent trauma particularly, surgical trauma in patients
with FAP (see above). A similar relationship has been observed in some
sporadically occurring desmoid tumors. In one series, an antecedent
history of trauma at the tumor site was elicited in 28 percent of 32
primary desmoid tumors
Abdominal desmoids tend to occur in women during or following
pregnancy. The classic presentation is that of an abdominal mass that is
separate from the uterus Trauma related to pregnancy and exposure to
elevated hormone levels may both be contributory. As an example, one case
report describes a desmoid tumor that developed at the site of a prior
cesarean section scar during a subsequent pregnancy . Subsequent pregnancy
is not necessarily a risk factor for recurrence or development of new
disease in a woman who develops a pregnancy-related desmoid
CLINICAL PRESENTATION AND
DIAGNOSIS
— Most desmoid tumors present as a painless or minimally painful mass with
a history of slow growth. Intraabdominal desmoids can be associated with
intestinal obstruction, mucosal ischemia, or functional deterioration in
an ileoanal anastomoses (typically in a patient who has undergone
colectomy for FAP
Desmoid tumors can develop at virtually any body site; they most
commonly arise in the torso (shoulder girdle and hip-buttock region) and
the extremities. The location is usually deep in the muscles or along
fascial planes. Desmoid tumors may be multifocal on an extremity, but they
rarely occur at different regions in the same patient.
Diagnosis —
Ultrasound is often the first method of examination of a soft tissue
lesion on the torso or extremity. If the mass is solid, CT or MRI are
needed to determine adherence to adjacent structures and resectability.
Although desmoids can be adequately evaluated by CT, we prefer MRI for
definition of the pattern and extent of involvement. There are no
radiographic characteristics that can reliably distinguish desmoids from
malignant soft tissue tumors.
The diagnosis of a desmoid tumor can only be established by
histological examination of a biopsy specimen. Incisional biopsy is often
preferred over a needle biopsy because of the need to distinguish between
a benign and malignant process with very high confidence.
There is no accepted staging system for desmoid tumors.
Natural history
— Although benign, desmoid tumors are locally infiltrative and can be
fatal by causing destruction of adjacent vital structures and organs. In a
report of 138 patients managed at one institution between 1965 and 1984,
11 died of their disease Factors associated with a poor outcome in this
study were: age 18 to 30 years, presentation with locally recurrent
disease, incomplete excision, and no postoperative radiation therapy (RT).
However, this series may not be representative since many patients had
advanced disease at the time of diagnosis. Other centers report an overall
mortality rate <1 percent in patients with desmoid tumors at other than
intraabdominal sites
Thus, even though desmoids are benign in the sense that they cannot
produce distant metastases, the disease process may be devastating, and
occasionally fatal. Fortunately, the pace of progression is usually
relatively slow, with periods of comparative stability or even temporary
regression. Regrowth is not an inevitable consequence following grossly
incomplete surgical resection
TREATMENT
— Treatment of desmoid tumors is indicated when they cause
symptoms, if there is imminent risk to adjacent structures, or if they
create cosmetic concerns.
Surgery —
Because of their locally infiltrative nature, desmoid tumors are treated
by surgical resection with a wide margin when medically and technically
feasible. Since these are benign tumors,
the treatment strategy should be
to obtain tumor-free margins using function-preserving approaches to
minimize major morbidity (functional and/or cosmetic).
Extraabdominal and abdominal wall desmoids are more often resectable
than are intraabdominal tumors. Surgery may be difficult and even
impossible for intraabdominal desmoids, although it may be an important
option for selected patients. Medical therapy may be considered as a
first-line option, particularly for tumors that involve the mesentery or
encase vessels and organs (see below).
Despite their benign character, desmoid tumors have a high rate of
recurrence with surgery alone. Even patients who undergo aggressive
resection with wide margins have recurrence rates of 23 to 39 percent When
they recur, salvage therapy with RT and/or repeat excision is usually
successful.
Intraabdominal desmoids that arise in the setting of Gardner's syndrome
have a greater tendency for local recurrence and multiple lesions.
Moreover, they may be relatively refractory to RT and may be rendered more
aggressive after surgical intervention . In one report, as an example,
tumor debulking led to aggressive progression of desmoids in four of six
patients This has led some clinicians to advocate conservative management
with noncytotoxic therapy as opposed to resection and/or RT in these
patients although this is controversial
Importance of resection
margins — The available
data are conflicting with regard to the importance of complete resection.
Some authors report that the risk of recurrence is independent of
margin status while others demonstrate higher recurrence rates with
close or positive resection margins As an example, in one series of
203 patients undergoing surgery for either primary or recurrent desmoid
tumors over a 35 year period, margins were microscopically positive in 57
and negative in 146. As expected, the disease-free survival rate was
significantly better in patients with primary disease (76 versus 59
percent at 10 years), but it was not significantly worse for those with
microscopically positive versus negative margins at primary surgery (five
year disease-free survival rate for those with positive and negative
margins, 79 versus 82 percent; at 10 years, 74 versus 77 percent).
These data have led some to conclude that aggressive attempts to
achieve negative resection margins are not warranted if they result in
excessive morbidity. Moreover, uncertainty as to the importance of
positive resection margins has led to controversy with regard to the
utility of postoperative RT for patients with incompletely resected
disease. These data are discussed below.
Radiation therapy
— RT is an effective primary therapeutic option for
patients who are not good surgical candidates, those who decline surgery,
and those for whom surgical morbidity would be excessive. The time to
regression after RT alone is often quite long and several years may elapse
before regression is complete
In a number of reports, RT
alone (50 to 60 Gy) or combined with surgery in patients with positive
resection margins achieves long-term control in approximately 70 to 80
percent of desmoids. The volume of disease does not appear to
affect the probability of local control.
The contribution of RT to the management of desmoid tumors can be
illustrated by the following reports:
- Our experience of 107 patients treated with surgery (n = 51), RT
alone (n =15), or surgery followed by RT (n = 41) suggests that RT is a
reasonable option for many patients. The five-year actuarial local
control rates were 69, 93, and 72 percent in the three groups,
respectively. The five year rate
of local control with surgery alone was 50 percent (three of six) for
gross residual disease, 56 percent for microscopically positive margins,
and 77 percent for negative margins. In contrast, local control was
achieved in all five patients treated with RT alone for a primary tumor,
and in 9 of 10 treated for recurrent lesions.
- Similar conclusions were reached in a comparative review of
published experience with treatment of desmoid tumors.
Local control rates after
surgery alone (n = 381) were 61 percent overall, and were 72 and 41
percent for those with negative or positive margins, respectively. For
patients undergoing surgery plus RT (n = 297), the local control rate
was 75 percent overall, and it was 94 and 75 percent for cases with
negative and positive margins, respectively. The overall local control
rate for RT alone (n = 102) was 78 percent, and was 83 and 73 percent
for those treated for primary or recurrent tumors, respectively.
- Remarkably similar local control rates (80 to 81 percent) were noted
in a German patterns of care study of 345 patients from 19 institutions
who were followed for a median of 43 months after either definitive RT
or surgery followed by RT.
The recommended dose of RT for
definitive therapy is 50 to 60 Gy in six to seven weeks at 1.8 to 2 Gy per
fraction. Local recurrence rates do not appear to be reduced by the
use of higher doses. In one study, for example, 23 patients were treated
with RT for unresectable disease; the relapse rate at five years was 31
percent. Radiation doses above 56 Gy did not improve outcome, and were
associated with more complications (30 versus 5 percent with lower doses
at 15 years). Positive resection margins were not an adverse prognostic
factor in this report.
Postoperative RT
— Postoperative RT is generally
not recommended for patients with uninvolved resection margins. The
benefit of postoperative RT for those with positive resection margins is
controversial. As noted previously, the status of the resection margins
has not been shown to significantly increase the risk of recurrence in
several retrospective series. Furthermore, even in those series that did
show a higher rate of recurrence in patients with positive margins in the
absence of RT, successful salvage therapy at the time of recurrence has
been possible in the majority of patients
These issues were illustrated in a retrospective analysis of 189
patients treated for desmoid tumors at MD Anderson Cancer Center, 122 with
surgery alone, 46 with surgery and RT, and 21 with RT alone. Ten-year
local recurrence rates were significantly higher in surgically treated
patients who had positive as compared to negative resection margins (54
versus 27 percent), and surgical margin status was the single most
significant determinant of recurrence in surgically treated patients.
Among the 40 patients with incompletely resected desmoids, local
recurrence rates were significantly lower if postoperative RT was added
(10-year recurrence rates 31 versus 54 percent). However, salvage
treatment with surgery or RT was successful in 43 of the 58 patients who
recurred (including 7 of the 10 with positive initial resection margins),
and no patients died of aggressive fibromatosis.
The benefit of RT plus surgery was further addressed in a comparative
review of published experience with treatment of desmoid tumors. Local
control rates after surgery alone (n = 381) were 61 percent overall, and
were 72 and 41 percent for those with negative or positive margins,
respectively. For patients undergoing surgery plus RT (n = 297), the local
control rate was 75 percent overall, and it was 94 and 75 percent for
cases with negative and positive margins, respectively. The overall local
control rate for RT alone (n = 102) was 78 percent, and was 83 and 73
percent for those treated for primary or recurrent tumors, respectively.
From these data, many clinicians (including our group) conclude that
local failure is not inevitable if residual tumor is left in situ.
Deferring radiation is an
acceptable option for patients with microscopically positive
margins as long as local progression, if it occurred, would not risk
significant morbidity. However, we and others recommend postoperative
irradiation for microscopically positive margins after resection of
recurrent disease, and for patients with gross or macroscopic residual
disease.
Others disagree, citing the importance of local control to cure of this
nonmetastatic condition, and the greater likelihood of local control with
either RT alone or surgery followed by RT for incompletely resected
disease. A prospective randomized trial is currently underway by the
American College of Surgeons Oncology Group (ACOSOG trial Z9031), in which
patients with retroperitoneal sarcomas (including desmoid tumors) are
randomly assigned to surgery with or without preoperative RT . Eligible
patients should be encouraged to participate.
Neoadjuvant therapy
— A novel approach to reducing the rate of local recurrence is the
use of neoadjuvant (preoperative) radiotherapy with or without
chemotherapy:
- In one small series,13 patients with potentially resectable desmoid
tumors received doxorubicin 30 mg by continuous infusion daily for three
days concurrent with RT (10 x 3 Gy), with resection performed four to
six weeks later With a median follow-up of 71 months, there were only
two local recurrences (15 percent).
- In another report, 58 patients underwent preoperative RT and
followed for a median of 69 months. There were 11 local recurrences (19
percent), and two patients had major wound complications (3.4 percent).
This rate was lower than the authors' historical experience with a
similar RT dose and schedule for soft tissue sarcomas, leading them to
speculate that patients with fibromatosis might have an inherent wound
healing advantage over those with soft tissue sarcoma.
While these results are promising, confirmation with larger,
prospective, randomized trials is needed.
Systemic therapy
— Patients with extraabdominal desmoids and multiple locoregional
recurrences despite adequate local therapy are generally considered for
systemic therapy. Other indications for systemic therapy include
unresectable tumors, and intraabdominal desmoids, especially those
associated with familial adenomatous polyposis (FAP). In such patients,
early and aggressive systemic therapy is important to avoid
life-threatening complications.
A variety of agents are active, including noncytotoxic therapy (NSAIDs,
hormone manipulation, pirfenidone (5-methyl-1-phenyl-2-(1H)-pyridone), an
antifibroblast agent) and cytotoxic chemotherapy. Most of the reported
data come from isolated case reports, limiting the conclusions that can be
drawn as to the relative effectiveness of these agents in the treatment of
desmoid tumors.
An important point is that clinical situations without any impending
threat to life or function can be treated with less toxic approaches, such
as hormone therapy or NSAIDs. Cytotoxic chemotherapy is a more appropriate
choice for patients with rapidly growing tumors, or those who are highly
symptomatic.
Noncytotoxic systemic therapy
— Clinical and experimental evidence suggest the hormone dependency
of desmoid growth. Particularly in patients who do not have good options
for surgery or radiation, therapy is often begun with a hormonal agent
such as tamoxifen; there is
less published experience with the antiestrogen toremifene, raloxifene, or
progestational agents
Clinical benefit (symptomatic improvement with shrinkage or
stabilization of disease) is reported in approximately 50 percent of
patients, with most of the objective responses being partial rather than
complete. Tumors are slow to manifest an actual reduction in size,
not infrequently, shrinkage lags behind discontinuation of therapy by
months or even years. Response durations range from seven months to 12
years
The mechanism underlying benefit is unclear since responses to
tamoxifen have been seen in desmoids that do not express hormone
receptors. The latency of the therapeutic response has led some to suggest
that the mechanism of action could be deprivation of a growth signal or
cytokine, which results in prolonged and continued regression long after
treatment discontinuation.
There are also documented responses to
NSAIDs (most often sulindac),
both alone and in combination with tamoxifen. Although response rates as
high as 70 percent are reported with combined therapy, regression is
usually partial and may take many months after an initial period of tumor
enlargement. In one report, 10 of 13 patients with FAP-associated desmoids
responded to daily tamoxifen (120 mg daily) and sulindac (300 mg daily),
as did two of eight recurrent sporadic tumors.
The contribution of tamoxifen to these results is unclear. At least one
report documents the resolution of a desmoid tumor being treated with
indomethacin and ascorbic acid for 14 months. NSAIDs alone represent a
potentially attractive treatment option, particularly since they also
appear to protect against colon cancer.
Several case reports describe objective response or prolonged periods
of disease stabilization with interferon alfa, in some cases following
failure of sulindac and tamoxifen
An important point is that many of the above clinical studies using
noncytotoxic agents predate the widespread use of objective response
criteria such as the RECIST criteria. Most experts treating large numbers
of these recurrent tumors find that the true objective regression rates
with these agents is in the range of 10 to 15 percent range with another
25 percent of patients experiencing minor shrinkage or tumor stabilization
(clinical benefit rate 50 percent).
Chemotherapy —
As noted previously, desmoid tumors, particularly those arising in the
setting of FAP, defy a prevalent dogma in clinical oncology that
slow-growing tumors without metastatic potential should not respond to
chemotherapy. In fact, several regimens are active, and in all cases,
durable response lasting years have been reported, clearly establishing
the efficacy of chemotherapy in this disease
Since randomized trials are not available, the optimal regimen is
uncertain . In general, an approach which matches the expected toxicity of
systemic therapy with the degree of symptoms caused by the tumor or the
rate of tumor growth appears to be the most reasonable.
Imatinib — At
least one case report suggests clinical and radiographic benefit from the
tyrosine kinase inhibitor imatinib (Gleevec®),
an effect that is presumably due to tumor expression of activated receptor
tyrosine kinases c-kit and/or platelet-derived growth factor receptor (PDGFR)
. A study of imatinib in 50 patients with recurrent desmoid tumors was
completed by the Sarcoma Alliance for Research through Collaboration, and
preliminary data presented at the 2004 meeting of the American Society of
Clinical Oncology. The two and four month the progression free survival
rates were 91 and 78 percent, respectively. Polymorphisms and mutations
were found in exon 18 of PDGFR-alpha in some patients. Maturation of these
data will be needed to determine the true clinical efficacy of imatinib in
patients with desmoid tumors.
Antifibrotic agents
— Antifibrotic agents seem
to represent a rational choice for therapy. Experience with one such
agent, pirfenidone, is limited to a pilot trial of 14 patients. Two of the
seven patients who continued therapy for at least 18 months had partial
tumor regression. This agent is not approved for use in the United States
or Europe.
Percutaneous chemical
ablation — At least one report suggests that percutaneous
injection of acetic acid into the desmoid can achieve objective tumor
shrinkage and prolonged periods of disease stabilization. Further
experience with this approach is needed.
SUMMARY AND RECOMMENDATIONS
— Desmoid tumors (also called aggressive fibromatosis) are benign slowly
growing fibroblastic neoplasms. Although desmoids do not have the capacity
to establish metastatic lesions they infiltrate adjacent tissues, causing
destruction of structures and/or organs that can be fatal. Nevertheless,
the likelihood of dying from aggressive fibromatosis is very low (<1
percent).
Treatment of desmoids is a clinical challenge. Although these are
benign tumors, because of their locally aggressive behavior and tendency
to relapse, multimodality treatment is often required, and best delivered
within the context of a multidisciplinary team specializing in sarcoma
treatment. Treatment recommendations should be based upon analysis of the
risk-to-benefit ratio because of the inconsistent nature and response to
treatment of this tumor.
- Surgical excision is the treatment of choice for patients with a
resectable lesion who are medically able to tolerate surgery. Even if
the gold standard is resection, there are data supporting a "wait and
see" strategy for stable, asymptomatic desmoids. We prefer initial
medical treatment in patients with desmoids in the setting of Gardner's
syndrome (see below).
- RT is an effective alternative if treatment is required for an
unresectable lesion or if surgery would result in major functional or
cosmetic defects.
- We do not recommend adjunctive RT in patients with primary desmoid
tumors who have microscopically positive margins. Since the incidence of
regrowth is low (ie, less than 50 percent), patients without recurrence
will be spared the long-term sequelae from radiation doses of 50 to 60
Gy. Furthermore, effective treatment is available if regrowth does
occur.
- For gross residual disease of a primary desmoid, we recommend a more
radical resection or RT. Due to the very high local control rates with
RT, more surgery is warranted only if there is strong confidence in
obtaining clear margins and functional loss is acceptable.
- For a recurrent desmoid tumor, our preference is surgical resection.
However, RT alone may be considered as the initial procedure in selected
patients due to the higher morbidity of repeat operation and the
increased probability of positive margins. We recommend postoperative RT
if the margins are positive.
- For patients with recurrent unresectable disease, it is reasonable
to begin treatment with tamoxifen or a low dose chemotherapy regimen
such as pegylated liposomal doxorubicin or the combination of
methotrexate plus vinorelbine. Standard doxorubicin-based regimens
should be reserved for refractory cases, patients with rapidly growiing
tumors, or those patients who are highly symptomatic.
- For patients with intraabdominal desmoids and Gardner's syndrome, we
prefer an initial trial of less toxic agents, such as sulindac with or
without tamoxifen. Surgery combined with radiation is the second option
if there is no response. In this situation, consideration should be
given to use of intraoperative electron beam therapy as a component of
the treatment.
- There is no standard protocol for follow-up of patients with desmoid
tumors. However, we typically follow patients by clinical examination
and radiographic studies (where appropriate) every six months for the
first two years, every 12 months to year six, and then biannually.
|