| With high dose chemoradiation the median
survival may be greater than 2 years as note below. Primary Analysis of the Phase II Component of a Phase I/II Dose Intensification Study Using Three-Dimensional Conformal Radiation Therapy and Concurrent Chemotherapy for Patients With Inoperable Non–Small-Cell Lung Cancer: RTOG 0117 Jeffrey D. Bradley,Journal of Clinical Oncology, Vol
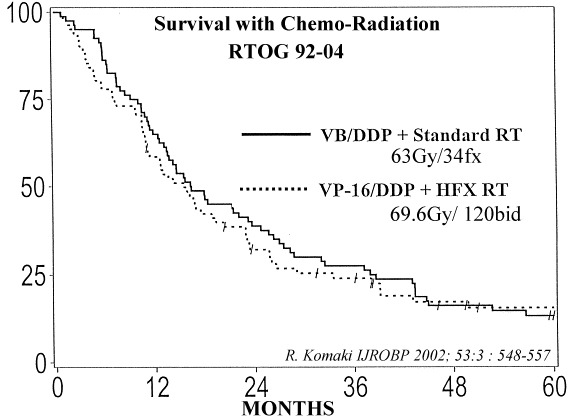
28, No 14 (May 10), 2010: pp. 2475-2480 Results Of the combined phase I/II enrollment, a total of 55 patients received 74 Gy, of whom 53 were evaluable. The median follow-up was 19.3 months for all patients and 25.4 months for those still alive. The median survival for all patients was 25.9 months. The percentage surviving at least 12 months was 75.5%. The median overall survival (OS) and progression-free survival (PFS) times for stage III patients (n = 44) were 21.6 months and 10.8 months, respectively. OS and PFS rates at 12 months were 72.7% and 50.0%, respectively. Twelve patients experienced grade ≥ 3 lung toxicity (two patients had grade 5 lung toxicity). Over the past several years, three other groups have prospectively tested the tolerability and efficacy of 74 Gy given with weekly paclitaxel and carboplatin chemotherapy. All three groups independently reached a similar conclusion: 74 Gy is likely the MTD in this setting. The North Central Cancer Treatment Group (NCCTG) has reported prospective phase I results for 13 patients treated to 70, 74, or 78 Gy. In their study, 70 and 74 Gy were well tolerated and 78 Gy was not. With a median follow-up of 28 months for the 13 patients on NCCTG N0028, the median survival time was 37 months. The Cancer and Leukemia Group B (CALGB) has also reported results of a randomized phase II comparison of two different chemotherapy regimens given with 74 Gy.Patients either received induction paclitaxel and carboplatin for two cycles followed by the same weekly chemotherapy or they received induction carboplatin and gemcitabine followed by twice weekly gemcitabine during RT. The trial enrolled 69 patients and had a median follow-up of 16.4 months at last report. The median survival was 24.2 months on the paclitaxel/carboplain arm. The gemcitibine arm was closed early because 13% of the patients had grade 5 pulmonary events. On the basis of these two trials as well as RTOG 0117, the NCCTG and the CALGB have joined efforts with the RTOG to support RTOG 0617 as an intergroup trial. North Carolina investigators have reported the results of four sequential prospective phase I/II studies to assess the safety and feasibility of high-dose (74-90 Gy) three-dimensional conformal radiation treatment in the setting of concurrent weekly carboplatin and paclitaxel chemotherapy.These investigators also delivered two cycles of carboplatin and paclitaxel neoadjuvantly before concurrent chemoradiotherapy. In total, 112 patients were accrued, with a median follow-up of 4.9 years for surviving patients. The median survival was 24 months. The 1-, 3-, and 5-year OS rates were 69%, 36%, and 24%, respectively. The relatively longer follow-up duration of this population provides information about late complication risks. Various studies have combined chemotherapy with radiation in various combinations. The RTOG 92-04 trial compared induction chemotherapy with concurrent chemoradiation as a standard dose or hyperfractionation, the 5-year overall and median survival rate was 13% and 16.4 months in Arm 1 compared with 16% and 15.5 months for Arm 2, respectively. Various other studies are also noted below |
|