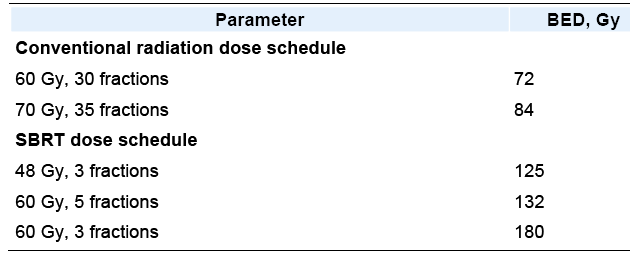
|
|||
|
Recent review (IJROBP 2011;79:660) had tables on: outcome, toxicity, dose constraints here and here ongoing research trials
are noted
here and RTOG 0618 and
RTOG 0813 Some studies are noted below |

Stereotactic radiotherapy for
primary lung cancer and pulmonary metastases: a noninvasive treatment approach in
medically inoperable patients.
Wulf J, Haedinger U, Oppitz U, Thiele W, Mueller G, Flentje M. Int J Radiat Oncol Biol
Phys. 2004 Sep 1;60(1):186-96.
Department of Radiotherapy, University of Wuerzburg,
The clinical results of dose escalation using stereotactic radiotherapy to increase local
tumor control in medically inoperable patients with Stage I-II non-small-cell lung cancer
or pulmonary metastases were evaluated. Twenty patients with Stage I-II non-small-cell
lung cancer and 41 patients with 51 pulmonary metastases not amenable to surgery were
treated with stereotactic radiotherapy at
3 x 10 Gy (n = 19), 3 x 12-12.5 Gy
to the
planning target volume enclosing 100%-isodose, with normalization to 150% at the
isocenter; n = 26) or 1 x 26 Gy to the planning target volume enclosing 80%-isodose (n =
26). The median follow-up was 11 months (range, 2-61 months) for primary lung cancer
patients and 9 months (range, 2-37 months) for patients with metastases. RESULTS: The
actuarial local control rate was 92% for lung cancer patients and 80% for metastasis
patients > or =1 year after treatment and was significantly improved by increasing
the dose from 3 x 10 Gy to 3 x 12-12.5 Gy or 1 x 26 Gy (p = 0.038). The overall survival
rate after 1 and 2 years was 52% and 32%, respectively, for lung cancer patients and 85%
and 33%, respectively, for metastasis patients, impaired because of systemic disease
progression. After 12 months, 60% of patients with primary lung cancer and 35% of patients
with pulmonary metastases were without systemic progression. No severe acute or late
toxicity was observed, and only 2 patients (3%) developed symptomatic Grade 2 pneumonitis,
which was successfully treated with oral steroids. CONCLUSION: Stereotactic radiotherapy
for lung tumors offers a very effective treatment option locally without significant
complications in medically impaired patients who are not amenable to surgery. Patient
selection is important, because those with a low risk of systemic progression are more
likely to benefit from this approach.
Stereotactic radiosurgery for lung tumors: preliminary report of a phase I trial.
Whyte RI, Crownover R, Murphy MJ, Martin DP, Rice TW, DeCamp MM Jr, Rodebaugh R,
Weinhous MS, Le QT. Ann Thorac Surg. 2003
Apr;75(4):1097-101.
Department of Cardiothoracic Surgery, Stanford University, Stanford,
California
Stereotactic radiosurgery is well established for the treatment of intracranial neoplasms
but its use for lung tumors is novel. METHODS: Twenty-three patients with biopsy-proven
lung tumors were recruited into a two-institution, dose-escalation, phase I clinical trial
using a frameless stereotactic radiosurgery system (CyberKnife). Fifteen patients had
primary lung tumors and 8 had metastatic tumors. The age range was 23 to 87 years (mean,
63 years). After undergoing computed tomography-guided percutaneous placement of two to
four small metal fiducials directly into the tumor, patients received
1,500 cGY of
radiation in a single fraction using a linear accelerator mounted on a
computer-controlled robotic arm. Safety, feasibility, and efficacy were studied. RESULTS:
Nine patients were treated with a breath-holding technique, and 14 with a
respiratory-gating, automated, robotic technique. Tumor size ranged from 1 to 5 cm in
maximal diameter. There were four complications related to fiducial placement: three
pneumothoraces requiring chest tube insertion and one emphysema exacerbation. There were
no grade 3 to 5 radiation-related complications. Follow-up ranged from 1 to 26 months
(mean, 7.0 months). Radiographic response was scored as complete in 2 patients, partial
in 15, stable in 4, and progressive in 2. Four patients died of non-treatment-related
causes at 1, 5, 9, and 11 months after radiation. CONCLUSIONS: Single-fraction
stereotactic radiosurgery is safe and feasible for the treatment of selected lung tumors.
Additional studies are planned to investigate the optimal radiation dose, best
motion-suppression technique, and overall treatment efficacy.
Extracranial stereotactic radioablation: results of a
phase I study in medically inoperable stage I non-small cell lung cancer.
Timmerman R, Papiez L, McGarry R, Likes L, DesRosiers C, Frost S, Williams M. Chest. 2003
Nov;124(5):1946-55.
Department of Radiation Oncology, Indiana University School of Medicine,
Indianapolis
Surgical resection is standard therapy for patients with stage I non-small cell lung
cancer (NSCLC), however, many patients are medically inoperable. We set out to investigate
a new therapy akin to brain radiosurgery called extracranial stereotactic radioablation
(ESR) in a phase I trial. Eligible patients included those with clinically staged T1
or T2 (tumor size, < or = 7 cm) N0M0 biopsy confirmed NSCLC. All patients had comorbid
medical problems that precluded thoracotomy. The median age was 75 years, and the median
Karnofsky performance status was 80. ESR was administered in three separate fractions over
2 weeks. Three to five patients were treated within each dose cohort starting at 800 cGy
per fraction (total, 2,400 cGy) followed by successive dose escalations of 200 cGy per
fraction (total increase per cohort, 600 cGy). Waiting periods occurred between dose
cohorts to observe toxicity. Patients with T1 vs T2 tumors underwent separate independent
dose escalations. RESULTS: A total of 37 patients were enrolled since February
2000. One patient experienced grade 3 pneumonitis, and another patient had grade 3
hypoxia. For the entire population, there was no appreciable decline in cardiopulmonary
function as measured by symptoms, physical examination, need for oxygen supplementation,
pulmonary function testing, arterial blood gas determinations, or regular chest imaging.
Both T-stage groups ultimately reached and tolerated 2,000 cGy per fraction for three
fractions (total, 6,000 cGy). The maximum tolerated dose for this therapy in either
T-stage group has yet to be reached. Tumors responded to treatment in 87% of patients
(complete response, 27%). After a median follow-up period of 15.2 months, six patients
experienced local failure, all of whom had received doses of < 1,800 cGy per fraction.
CONCLUSIONS: Very high radiation dose treatments were tolerated in this population of
medically inoperable patients with stage I NSCLC using ESR techniques.
Stereotactic body frame based fractionated radiosurgery on consecutive days for primary or
metastatic tumors in the lung.
Lee SW, Choi EK, Park HJ, Ahn SD, Kim JH, Kim KJ, Yoon SM, Kim YS, Yi BY. Lung Cancer. 2003
Jun;40(3):309-15.
Department of Radiation Oncology, Asan Medical Center, College of Medicine,
University of Ulsan, Seoul
To evaluate the feasibility and treatment outcomes of stereotactic radiosurgery (SRS)
using a stereotactic body frame (Precision Therapy), we prospectively reviewed 34
tumors of the 28 patients with primary or metastatic intrathoracic lung tumors.
Eligible patients included were nine with primary lung cancer and 19 with metastatic
tumors from the lung, liver, and many other organs.
A single dose of 10 Gy to the
clinical target volume (CTV) was delivered to a total dose of 30-40 Gy with three to four
fractions. Four to eight coplanar or non-coplanar static fields were generated to
adequately cover the planning target volume (PTV) as well as to exclude the critical
structures as much as possible. More than 90% of the PTV was delivered the prescribed dose
in the majority of cases (average; 96%, range; 74-100%). The mean PTV was 41.4 cm(3)
ranging from 4.4 to 230 cm(3). Set-up error was within 5 mm in all directions (X, Y, Z
axis). The response was evaluated by using a chest CT and/or 18FDG-PET scans after SRS
treatment, 11 patients (39%) showed complete response, 12 (43%) partial response
(decrease of more than 50% of the tumor volume), and four patients showed minimally
decreased tumor volume or stable disease, but one patient showed progression disease. With
a median follow-up period of 18 months, a local disease progression free interval was
ranging from 7 to 35 months. Although all patients developed grade one radiation
pneumonitis within 3 months, none had symptomatic or serious late complications after
completing SRS treatment. Given these observations, it is concluded that the stereotactic
body frame based SRS is a safe and effective treatment modality for the local management
of primary or metastatic lung tumors. However, the optimum total dose and fractionation
schedule used should be determined after the longer follow-up of these results.
Clinical outcomes of stereotactic radiotherapy
for stage I non-small cell lung cancer using a novel irradiation technique: patient
self-controlled breath-hold and beam switching using a combination of linear accelerator
and CT scanner.
Onishi H, Kuriyama K, Komiyama T, Tanaka S, Sano N, Marino K, Ikenaga S, Araki T,
Uematsu M. Lung Cancer. 2004 Jul;45(1):45-55.
Department of Radiation Oncology, Yamanashi Medical University, Japan
We have developed a novel irradiation technique for lung cancer that combines a linear
accelerator and CT scanner with patient-controlled breath-hold and radiation beam
switching. We applied this technique to stereotactic three-dimensional (3D) conformal
radiotherapy for stage I non-small cell lung cancer (NSCLC) and evaluated the primary
therapeutic outcomes. A total of 35 patients with stage I (15 IA, 20 IB) primary
NSCLC (20 adeno, 13 squamous cell, and 2 others) were treated with this technique.
Patients ranged from 65 to 92 years old (median, 78 years). Twenty-three (66%) patients
were medically inoperable due to mainly chronic pulmonary disease or high age.
Three-dimensional treatment plans were made using 10 different non-coplanar dynamic arcs. The
total dose of 60 Gy was delivered in 10 fractions (over 5-8 days) at the minimum dose
point in the planning target volume (PTV) using a 6 MV X-ray. After adjusting the
isocenter of the PTV to the planned position by a unit comprising CT and linear
accelerator, irradiation was performed under patient-controlled breath-hold and radiation
beam switching. All patients completed the treatment course without complaint. Complete
response (CR) and partial response (PR) rates were 8/35 (23%) and 25/35 (71%),
respectively. Pulmonary complications of National Cancer Institute-Common Toxicity
Criteria grade >2 were noted in three (9%) patients. During follow-up (range, 6-30
months; median, 13 months), two (6%) patients developed local progression and five (14%)
developed distant or regional lymph node metastases.
Two-year overall survival rates
for total patients and medically operable patients were 58 and 83%, respectively. In
conclusion, this new irradiation technique, utilizing patient-controlled radiation beam
switching under self-breath-hold after precise alignment of the isocenter, allows safe
high-dose stereotactic radiotherapy with sufficient margins around the CTV and reduced
treatment times. Based on the initial results, excellent local control with minimal
complications is expected for stage I NSCLC.
Clinical outcomes of 3D conformal
hypofractionated single high-dose radiotherapy for one or two lung tumors using a
stereotactic body frame.
Nagata Y, Negoro Y, Aoki T, Mizowaki T, Takayama K, Kokubo M, Araki N, Mitsumori M,
Sasai K, Shibamoto Y, Koga S, Yano S, Hiraoka M. Int J Radiat Oncol Biol
Phys. 2002 Mar 15;52(4):1041-6.
Department of Therapeutic Radiology and Oncology, Kyoto University Graduate
School of Medicine, Sakyo, Kyoto, Japan.
PURPOSE: This study was performed to evaluate the clinical outcomes of three-dimensional
(3D) conformal hypofractionated single high-dose radiotherapy for one or two lung tumors
using a stereotactic body frame. MATERIALS AND METHODS: Forty patients who were treated
between July 1998 and November 2000 and were followed for >10 months were included in
this study. Of the 40 patients, 31 had primary lung cancer and 9 had metastatic
lung cancer. The primary lung cancer was staged as T1N0M0, T2N0M0, and T3N0M0 in 19, 8,
and 4 patients, respectively. The primary sites of metastatic lung cancer were the colon
in 4, tongue in 2, and osteosarcoma, lung cancer, and hepatocellular carcinoma in 1 each.
3D treatment planning was performed to maintain the target dose homogeneity within 15% and
to decrease the irradiated lung volume from >20 Gy to <25%. All patients were
irradiated using a stereotactic body frame and received
4 times 10-12 Gy single
high-dose radiation at the isocenter during a period of 5-13 days (median 12).
RESULTS: The initial 3 patients received 40, and the remaining 37 patients received 48 Gy
after dose escalation. Of the 33 tumors followed >6 months, 6 tumors (18%) disappeared
completely after treatment. Twenty-five tumors (76%) decreased in size by 30% or more
after treatment. Therefore, 31 tumors
(94%) showed a local response. During the
follow-up of 4-37 months (median 19), no pulmonary complications greater than National
Cancer Institute-Common Toxicity Criteria Grade 2 were noted. Of the 16 patients with
histologically confirmed T1N0M0 primary lung cancer who received 48 Gy, all tumors were
locally controlled during the follow-up of 6-36 months (median = 19). In 9 tumors with
lung metastases that were irradiated with 48 Gy in total, 2 tumors did not show a local
response. Finally, 3 tumors (33%) with lung metastases relapsed locally at 6-12 months
(median 7) after treatment during the follow-up of 3-29 months (median 18). CONCLUSION: 3D
conformal hypofractionated single high-dose radiotherapy of
48 Gy in 4 fractions using a stereotactic body frame was useful for the treatment of lung tumors.
|
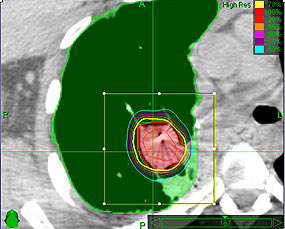
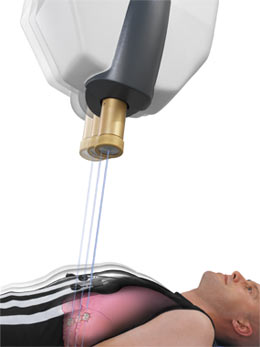 Radiosurgery
for Lung Cancer
may control up to 97% of lung cancers
Radiosurgery
for Lung Cancer
may control up to 97% of lung cancers