|
|
Stereotactic Body Radiotherapy for Localized Prostate Cancer: Interim Results of a Prospective Phase II Clinical Trial
King. IJROBP 2009;73:1043
Forty-one low-risk prostate cancer patients with 6 months' minimum follow-up received 36.25 Gy in five fractions of 7.25 Gy with image-guided SBRT alone using the CyberKnife. The early (<3 months) and late (>6 months) urinary and rectal toxicities were assessed using validated quality of life questionnaires (International Prostate Symptom Score, Expanded Prostate Cancer Index Composite) and the Radiation Therapy Oncology Group (RTOG) toxicity criteria. Patterns of prostate-specific antigen (PSA) response are analyzed. The median follow-up was 33 months. There were no RTOG Grade 4 acute or late rectal/urinary complications. There were 2 patients with RTOG Grade 3 late urinary toxicity and none with RTOG Grade 3 rectal complications. A reduced rate of severe rectal toxicities was observed with every-other-day vs. 5 consecutive days treatment regimen (0% vs. 38%, p = 0.0035). A benign PSA bounce (median, 0.4 ng/mL) was observed in 12 patients (29%) occurring at 18 months (median) after treatment. At last follow-up, no patient has had a PSA failure regardless of biochemical failure definition. Of 32 patients with 12 months minimum follow-up, 25 patients (78%) achieved a PSA nadir ≤0.4 ng/mL. A PSA decline to progressively lower nadirs up to 3 years after treatment was observed.
Conclusions
The early and late toxicity profile and PSA response for prostate SBRT are highly encouraging. Continued accrual and follow-up will be necessary to confirm durable biochemical control rates and low toxicity profiles.
In the late 1960s through early 1980s, motivated primarily by economy of resources, a clinical program was open in the United Kingdom delivering hypofractionated radiotherapy for prostate cancer (36 Gy in six fractions over 3 weeks). Although staging was limited (this was the pre–prostate-specific antigen [PSA] era), radiotherapy techniques were simple (this was the pre–computed tomography era) and many of these patients had high-risk features by today's criteria (e.g., bulky palpable disease or high grade), the update of that clinical experience with 22 years' follow-up confirmed the long-term safety and potential effectiveness of this treatment.
Modern understanding of the radiobiology of prostate cancer now offers a biologic rationale in favor of such a hypofractionated radiotherapy course (i.e., large dose per fraction) over a conventionally fractionated one (i.e., 1.8–2 Gy). The first study to suggest that prostate cancer possesses a radiobiology uniquely different from other cancers showed that one could quantify the sensitivity of prostate cancer to dose per fraction by comparing the dose response with permanent low-dose-rate brachytherapy to that from fractionated external beam. Using a standard radiobiologic model of dose response (the linear quadratic model), this study showed that prostate cancer possessed an unusually low α/β ratio of ∼1.5 Gy (i.e., a high sensitivity to dose per fraction). This α/β ratio is low compared with the value of ∼10 Gy for other cancers, and is also remarkably lower than that of late effects for normal tissues, where it is ∼3–5 Gy. The implications of such a high sensitivity to dose per fraction were immediately recognized, being that hypofractionation would be a more effective dose regimen for prostate cancer
Numerous studies have since followed the initial report of a low α/β ratio for prostate cancer. A recent review of 17 such studies estimated a mean α/β ratio of 1.85 Gy. There are four contemporary clinical series using external beam hypofractionated regimens, with dose per fraction ranging from 2.5 to 3.1 Gy and one using a linac-based stereotactic body radiotherapy (SBRT) technique delivering 5 daily fractions of 6.7 Gy. There are also several series using high-dose-rate brachytherapy combined with conventionally fractionated external beam with dose-per-fraction ranging from 5.5 Gy to 11.5 Gy and one with high-dose-rate brachytherapy monotherapy delivering eight to nine fractions of 6 Gy each. These clinical series have uniformly demonstrated excellent biochemical control rates and low rectal and bladder toxicities with the use of hypofractionated radiotherapy.
Fowler proposed several hypofractionated dose regimens for prostate cancer based on the assumption of a low α/β ratio. They showed that a significantly higher therapeutic ratio (i.e., simultaneous higher rates of tumor control rates and lower incidence of toxicities) could be achieved with these dose regimens. Although none is proposed as optimal, the gain in therapeutic ratio is proportional to the dose-per-fraction size. In this report, we present our preliminary experience of an ongoing prospective Phase II clinical trial using SBRT for localized low-risk prostate cancer that delivers 36.25 Gy in five fractions of 7.25 Gy, focusing on the early and late rectal/bladder toxicities as well as the initial patterns of PSA response.
Treatment specifics
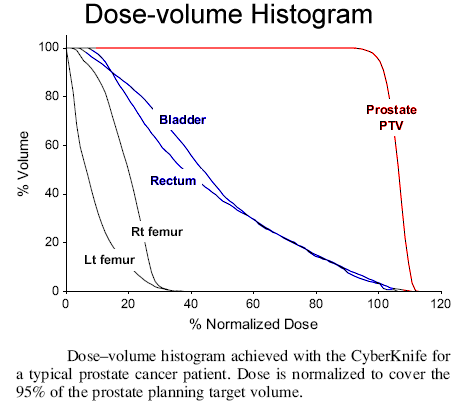
The Cyberknife (Accuray Inc., Sunnyvale, CA) was used to deliver image-guided SBRT. Three gold fiducials were placed in the prostate via transrectal ultrasound guidance. A same-day computed tomography scan was obtained with patients in the supine position and in an alpha cradle, at 1.25-mm slice thickness and indexing. Anatomic contouring of the prostate, seminal vesicles, rectum, bladder, penile bulb, and femoral heads were done. Dose was prescribed to the planning target volume that consisted of a volumetric expansion the prostate by 5 mm, reduced to 3 mm in the posterior direction. For the prescription dose to cover 95% of the planning target volume, normalization was required to the 89–90% isodose line (i.e., the resulting dose heterogeneity was 10–11%). we show a typical dose–volume histogram. In arriving at an optimal treatment plan, great care was taken to respect the rectal tolerance, which is particularly important when delivering hypofractionated radiotherapy. Our rectal dose–volume histogram goals were <50% rectal volume receiving 50% of the prescribed dose, <20% receiving 80% of the dose, <10% receiving 90% of the dose, and <5% receiving 100% of the dose. The course of radiotherapy consisted of five fractions of 7.25 Gy for a total dose of 36.25 Gy. Treatments were given over 5 consecutive days for the first 21 patients and 3 times per week subsequently. From the linear quadratic equation, one can derive the equivalent biologic dose when given at 2 Gy per fraction (EQD2) from that of a hypofractionated course for any tissue or tumor type by the simple relationship: EQD2 = D[α/β +d]/[α/β+2], where D is the total dose given at dose d, the dose per fraction. Our hypofractionated dose regimen corresponds to a tumor EQD2 of 90.6 Gy (assuming an α/β of 1.5 Gy), a normal tissue late effect EQD2 of 74.3 Gy (assuming an α/β of 3 Gy), and an acute toxicity EQD2 of 52.2 Gy (assuming an α/β of 10 Gy).
Discussion
Urinary/rectal toxicity and QOL
The outcomes from this clinical trial demonstrate that a hypofractionated course of stereotactic radiotherapy for localized prostate cancer is associated with urinary and rectal toxicities that are of the expected nature and severity as those experienced with conventionally fractionated courses of external beam radiotherapy. There was no severe urinary toxicity (RTOG Grade 4), and the 2 patients who experienced the worst problems were those who at baseline had described their urinary QOL as “mostly dissatisfied/unhappy.” Interestingly, after peaking at around 3 months, most patients returned to near-baseline levels of urinary satisfaction, and many have in fact improved above baseline levels at 2 years. An increase in the use of medications (e.g., alpha-blockers) could be an explanation for this observation, although we cannot exclude the possibility that it results from a late response from hypofractionated radiation therapy. We have not prospectively tracked the usage and timing of alpha-blockers in relation to urinary symptoms to be able to draw a more definitive conclusion. Our trial has now been modified to do so.
We compare our late urinary and rectal toxicities with that from the MD Anderson (MDA) dose-escalation trial that delivered 78 Gy in 2 Gy per fraction using three-dimensional conformal techniques. These toxicity rates are summarized. Although the incidence of low-grade (RTOG Grades 1 and 2) late urinary toxicity is about double that observed from the MDA dose-escalation trial, it has not resulted in a significant degradation of patients' urinary QOL. An evolving refinement of our technique to improve the dosimetry with the CyberKnife by using a urethral “tuning” structure to limit the dose heterogeneity from encroaching onto the urethra will likely lessen this toxicity in the future.
In comparing our results, we note that the MDA data are of a much longer median follow-up time of 8.7 years. In addition, their study showed an actuarial increase in toxicity with time achieving a plateau at around 5 years. Because our follow-up is much shorter, we must remain cautious about the interpretation of our late urinary and rectal toxicities because it is fully expected that these will continue to appear at least up to 5 years after treatment. We also note that comparison with the MDA results assumes that our patients had a similar baseline QOL profile as theirs.
There were no severe rectal toxicities (RTOG Grade 3 or 4) observed. A decline in patients' rectal QOL score appears to plateau around 3 months after RT, persisting at the “very small/small problem” up to 2 years after radiation therapy. No significant difference in the incidence of low-grade rectal toxicity (RTOG Grades 1 and 2) was observed when compared with the MDA dose-escalation trial.
QD vs. QOD
Our data allowed us to study differences in late toxicities between patients treated over 5 consecutive days (QD) and those treated every other day (QOD). A significant improvement was observed for late rectal problems when treatment was given QOD, where 0/20 patients reported a score of 4 or 5 compared with 8/21 patients when treated QD (p = 0.0035) for any rectal symptom, and 0/20 vs. 5/21, respectively, for overall rectal QOL (p = 0.048). Although fewer patients experienced a QOL score 4–6 for late urinary problems with QOD vs. QD treatment, 1/20 vs. 4/21, it was not significant (p = 0.34). The apparent improvement in rectal toxicity with QOD vs. QD regimen, if real, is interesting for what it suggests about the repair kinetics of hypofractionated radiation damage to the rectum. The data for late bladder and rectal toxicity suggests a repair half-life of ∼1 h (e.g., reference). Thus after 24 h, the repair of sublethal damage is complete (it should be nearly complete after five half-lives) and no further gain (or reduced toxicity) would be observed with a longer interval between dose fractions. One possible explanation of our observations is for a much longer repair half-life, on the order of at least 8 h, because repair is incomplete by 24 h but approximately complete by 48 h. This seems unlikely because it is inconsistent with previous data on repair kinetics. Other possible explanations are that either we are seeing the effects of normal tissue repopulation of rectal mucosa or that late damage actually results from vascular injury. Although these are only hypotheses, it is possible that either a separate mechanism of repair for late rectal effects or a different nature of radiation damage is present with hypofractionation. We are cautious about overinterpreting this data, but given our observations, we favor treating with a longer interval between fractions for hypofractionated dose regimens. We note that only a randomized trial would be able to properly study differences between QD and QOD SBRT regimens.
PSA response
The patterns of PSA response from our trial are highly encouraging. It is interesting to note the high proportion of patients (78%) with 12 or more months of follow-up achieving a low PSA nadir of 0.4 ng/mL. It is also worth noting that the PSA nadir achieved is progressively lower as time goes by, up to 3 years. This continued late PSA response after radiation therapy for prostate cancer is well-known and is consistent with the radiation biology of prostate cancer behaving similarly to that of late effects in normal tissues. What if our radiobiologic hypothesis for prostate cancer is wrong and that it in fact possesses an α/β ratio that is similar to other tumors (i.e., ∼10 Gy)? In that case, the tumor dose from our hypofractionated regimen, EQD2 = 52 Gy, would be seriously inadequate. An estimate of the 5-year biochemical control rate based on the dose response for low-risk prostate cancer is predicted to be only ∼40% for 52 Gy, as opposed to ∼90% for 78 Gy. Although our follow-up time is relatively brief, we have not observed a biochemical failure so far.
We have also shown that a benign PSA bounce was present after hypofractionated radiation therapy at roughly the same frequency, timing, and magnitude as has been described after permanent brachytherapy or after external beam radiotherapy
Conclusion
This study suggests that hypofractionated radiotherapy for localized prostate cancer has an early and late toxicity profile no worse than with dose-escalated radiotherapy delivered at conventional fractionation. The favorable biochemical response observed supports the radiobiologic assumption on which the rationale for prostate cancer hypofractionation is based. Continued pursuit of this trial seems warranted but with reasonable caution however, because longer follow-up will be necessary to confirm durable biochemical control rates and low late toxicity profiles.