Sixty-one women underwent radiation therapy with curative intent for IBC
between 1982 and 2001. All but five women received trimodality therapy. Neoadjuvant
chemotherapy was given to the majority of women (n = 43 patients), although some received
up-front surgery as first therapy (n = 18 patients).
RESULTS
With a median potential observation time after diagnosis of 14 years, freedom from
locoregional disease progression was 78%, freedom from distant metastasis was 45%, and
the cause-specific survival rate was 47% at 5 years. Approximately 40% of the 56
patients who received trimodality therapy remained free of disease. Multivariate analysis
demonstrated three factors that were found to be associated significantly with improved
cause-specific survival: pathologic tumor size < 4 cm , up-front surgery , and local
disease control . Factors that were found to be associated with better freedom from
locoregional disease progression were pathologic tumor size (< 4 cm; P = 0.0157), age
(> 55 years; P = 0.0596), and radiation dose ( 60 grays [Gy]; P = 0.0621).
CONCLUSIONS
IBC is an aggressive disease that is treated effectively in select patients by
multimodality therapy. Patient outcomes may be improved with therapies that result in
better local and systemic control. Further studies are warranted to address the optimal
sequence of trimodality therapy and the optimal administration of each agent.
Cancer
2004;100:920-8.DISCUSSION
In this series of patients with IBC, we report the efficacy of multimodality therapy and
identify a number of clinical and treatment-related factors that require special attention
for the appropriate diagnosis and optimal treatment of IBC. Multivariate analysis
demonstrated that the following three factors were associated with improved survival:
smaller pathologic tumor size, local disease control, and up-front surgery. Of particular
interest is the latter, which suggests an improved outcome for a sequence of therapy that
deviates from the widespread practice of neoadjuvant chemotherapy. The optimal role of
surgery in the treatment of IBC is difficult to interpret from the literature. Reports
that include both surgery and RT have shown either no survival benefit or a possible
benefit in combination with chemotherapy. Given favorable reported outcomes for
alternating chemotherapy and RT, and given similar outcomes when either RT or surgery
follows chemotherapy, even the necessity of surgery has been questioned.Meanwhile, the
timing of surgery has not been defined. At the University of Florida, several patients
underwent mastectomy at the time of initial presentation to decrease tumor burden
expeditiously. For reasons that are not entirely clear, those patients had a better
outcome compared with patients who were treated initially with chemotherapy, as noted on
previous analysis. It is noteworthy that this difference could be demonstrated only on
multivariate analysis, which controlled for potentially confounding factors. It is
conceivable that leaving a large source of tumor cells in place during neoadjuvant
chemotherapy actually permits the seeding and growth of more metastases in the face of
chemotherapy, such that initial surgery is more effective. Although the addition of
chemotherapy certainly has improved treatment outcomes overall for patients with breast
carcinoma, it is important to note that, in patients with IBC and with locally advanced
breast carcinoma, chemotherapy is only moderately effective in eliminating distant
disease, because 50% of patients succumb to breast carcinoma with distant metastases.
It also is important to note that randomized trials that have analyzed the potential
benefit of neoadjuvant chemotherapy have failed to show a decided survival advantage and
have suggested a slight decrease in local disease control with a delay of locoregional
treatment.
The number of prognostic factors analyzed in the current series may be larger than any
other study has reported. The issue of pathologic dermal lymphatic invasion merits special
mention. Uncertainty exists regarding the significance of a pathologically based diagnosis
of IBC. Because of sampling heterogeneity, dermal lymphatic invasion may not be evident in
all cases; it is estimated that it can be identified in only up to 75% of patients.
Consequently, the diagnosis of IBC is permitted based on clinical criteria alone. In turn,
the classification of disease in which dermal lymphatic invasion is observed in the
absence of clinical features (occult IBC) is not clear. Many report outcomes similar to
the outcomes reported in patients with classic IBC, although some have described occult
IBC as a more benign process, which may reflect the notion that noninflammatory breast
carcinomas (e.g., locally advanced breast carcinoma) can have incidental tumor emboli in
the dermal lymphatics. In the current series, roughly 70% of patients had documented
pathologic dermal lymphatic invasion, and 18% of patients had occult IBC. There was no
detectable difference in outcome for patients with dermal lymphatic invasion or clinically
occult IBC. Based on these results, no recommendations can be made to treat these patients
differently from the currently accepted standard of care for patients with IBC.
The presumed benefit of trimodality therapy is both improved local disease control and
survival. It is intuitive that successful local control would be associated with improved
survival, because local failures generally are not salvageable. The 20% local failure rate
in all patients who received surgery and RT indicates room for improvement. With the
exception of type of chemotherapy (which likely was confounded by imbalances in pathologic
tumor size and delay of effective locoregional treatment), RT dose was the only other
factor that was found to be subject to treatment modification. Hyperfractionated RT was
not administered routinely, because it generally was reserved for patients whose response
to neoadjuvant chemotherapy was insufficient to render the tumor operable. Theoretically,
the use of hyperfractionation enables dose escalation and can be considered the radiation
counterpart of dose density chemotherapy. Intuitively, hyperfractionated irradiation
should be effective, particularly in rapidly proliferating tumors, such as classic IBC. An
early report from the M. D. Anderson Cancer Center suggested potential benefits that were
confirmed in a later study, leading to improved local control. IBC
patients at our institution currently are treated with hyperfractionated RT up to doses of
66 Gy.
Because of the low incidence of IBC compared with other invasive breast carcinomas,
prospective controlled trials to define optimal management strategies are not feasible.
Thus, retrospective studies are essential to continued progress in the management of this
disease. In part because of limited numbers for analysis and patient heterogeneity, some
caution must be exercised in the interpretation of their results. The findings of this
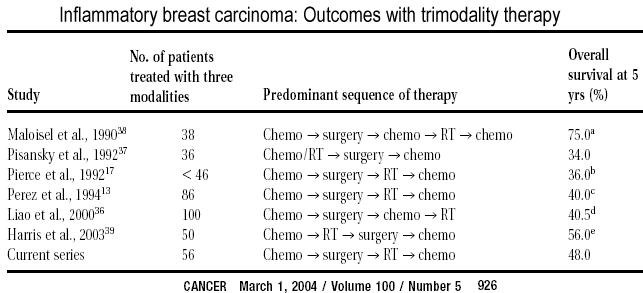
series compare favorably with other published reports. Table above includes series
that have reported overall survival for > 30 patients who were treated with trimodality
therapy for IBC. Overall survival was reported to be between 34-75% at 5 years. In the
literature, several prognostic factors have been associated with better survival in
patients with IBC, including better response to neoadjuvant chemotherapy, early response
to neoadjuvant chemotherapy,more chemotherapeutic agents, no or limited clinical or
pathologic lymph node involvement, premenopausal status and younger age, positive hormone
receptor status, the absence of peau d'orange or diffuse erythematous involvement at
presentation, any palpable mass at presentation, smaller pathologic tumor size, the
absence of distant metastases at presentation, the inclusion of mastectomy as part of
treatment, negative surgical margin status, local control,and higher RT dose. Both the
patient heterogeneity and the small numbers in each of the studies most likely have led to
the lack of uniformly reported prognostic factors. For these reasons, the current series
also was unable to confirm improved survival with any reported factors other than
pathologic tumor size and freedom from locoregional disease progression. |