A Phase I Dose-Escalation Study of Fractionated Stereotactic Radiosurgery in Combination With Gefitinib (Iressa) in Patients With Recurrent Malignant GliomasS chwer. IJROBP 2007;70:993To determine the maximum tolerated dose (MTD) of fractionated stereotactic radiosurgery (SRS) with gefitinib in patients with recurrent malignant gliomas. Eligible patients had pathologically proved recurrent anaplastic astrocytoma or glioblastoma. Patients started gefitinib (250 mg/day) 7 days before SRS and continued for 1 year or until disease progression. SRS was delivered in three fractions over 3 days. The planning target volume (PTV) was the T1-weighted MRI postcontrast enhancing lesion + 2 mm. The first cohort received an SRS dose of 18 Gy, and subsequent cohorts received higher doses up to the maximum dose of 36 Gy. Dose-limiting toxicity (DLT) was any Grade 3 toxicity. The MTD was exceeded if 2 of 6 patients in a cohort experienced DLT. SRS techniquePatients underwent a treatment planning computed tomography (CT) study with 3-mm slices. CT/MRI fusions were performed, and the planning target volume (PTV) was defined as the contrast-enhancing lesion plus a 2-mm margin on the basis of the brain MRI T1-weighted images. Patients were immobilized using a custom-molded removable head frame that provided reproducibility within 2 mm. Treatments were delivered with a dedicated linear accelerator-based treatment planning and micro-multileaf delivery system (BrainSCAN software and Novalis system, BrainLAB, Heimstetten, Germany). Single isocenter treatment plans were accomplished in all patients. All SRS plans were optimized according to the RTOG SRS quality assurance guidelines to ensure maximal dose conformity at the tumor margin and to attempt to achieve a rapid dose falloff toward the critical structures. SRS was delivered using either three to five dynamic arcs or five to seven static beams with 6-MV photons. The dose was prescribed to the specific isodose line that best encompassed the PTV. SRS was delivered in three fractions over 3 consecutive days. The lens and cervical spine were shielded from the direct beam at all times. Attempts were made to limit the total radiation dose to the optic chiasm to 22 Gy, the retina of at least one eye to 20 Gy, and the brainstem to 25 Gy. ResultsCharacteristics of the 15 patients enrolled were: 9 men, 6 women; median age, 47 years (range, 23–65 years); 11 glioblastoma, 4 AA; median prior RT dose, 60 Gy (range, 54–61.2 Gy); median interval since RT, 12 months (range, 3–57 months); median PTV, 41 cc (range, 12–151 cc). Median follow-up time was 7 months (range, 2–28 months). Median time on gefitinib was 5 months (range, 2–12 months). No patient experienced a DLT, and the SRS dose was escalated from 18 to 36 Gy. Grade 1–2 gefitinib-related dermatitis and diarrhea were common (10 and 7 patients, respectively). Conclusion: Fractionated SRS to a dose of 36 Gy in three fractions is well tolerated with gefitinib at daily dose of 250 mg. Further studies of SRS and novel molecular targeted agents are warranted in this challenging clinical setting.
|
|
|
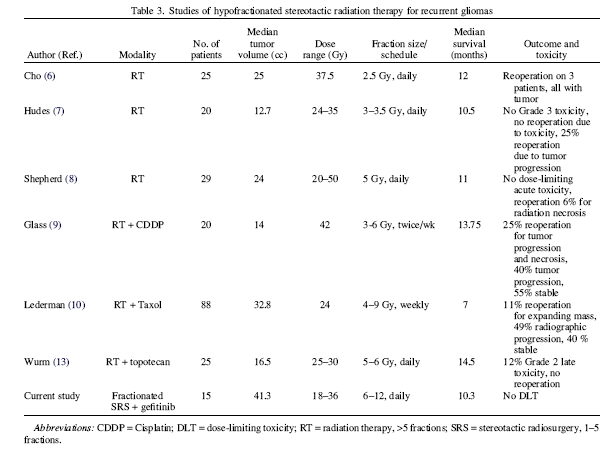
DiscussionResults of this study indicate that a total SRS dose of 36 Gy in three fractions may be safely administered in combination with gefitinib, at a dose of 250 mg per day, to patients with a recurrent malignant glioma after initial RT (54–61.2 Gy) and chemotherapy. To our knowledge, this study represents the first formal prospective trial in which SRS has been combined with an EGFR-TKI. There is ongoing debate about whether the linear quadratic model can be accurately applied to model the biological effect of single fraction doses in the range typically applied for single-fraction SRS and the multiple-fraction SRS used in this study. Regarding this, Guerrero and Li suggested accommodation for the expected intrafractional repair during an individual high-dose treatment by the use of the Curtis lethal–potentially lethal formalism. Thus it is difficult to compare the relative biological potency of the doses used in our study with the single fraction SRS doses established as safe in the RTOG 90-05 Phase I study. In that particular study, which included both previously irradiated primary brain tumors (median prior radiation dose, 60 Gy) and brain metastases (median prior dose, 30 Gy), the MTD for recurrent tumors of maximum diameters ≤2 cm, 2.1–3 cm, and 3.1–4 cm were 24 Gy, 18 Gy, and 15 Gy, respectively. The biologic equivalent dose (BED) of the highest of these doses (24 Gy in a single fraction), assuming an α/β of 10 Gy for tumor tissue and applying the standard BED equation would be 82 Gy-10. The BED of 36 Gy in three fractions, determined with the same assumptions, would be 79.2 Gy. Besides the fact that the linear quadratic–based BED does not account for the mitigating effect of intrafractional repair, there is also the problem of reconciling differences in dose inhomogeneity within the target volume, which are not fully represented by the simple expression of prescription dose to the periphery of the PTV. Yet another difference between our study and the RTOG 90-05 study is the volume of tumors treated. Specifically, here, tumors up to 6 cm in maximum diameter were allowed, whereas the RTOG 90-05 study limited the maximum tumor dimension to 4 cm. Volumetric data about the PTV were not included in the RTOG 90-05 report, and therefore additional comparison between the volumes of tumors treated on the current study and the RTOG 90-05 study is not possible. In the last decade, several groups had reported their data on FSRT in patients with recurrent malignant gliomas who received prior radiation to a median dose around 60 Gy. Those studies were mostly retrospective in nature, and the RT doses and fractionation schemes vary significantly (the RT doses ranged from 24 Gy to 50 Gy, and fraction size from 2.5–10 Gy). The median tumor volumes treated were in the range of 12.7 cc to 33 cc. Across those various fractionation and dose schemes, the treatment regimens were reportedly well tolerated, with reoperation rates between 0% and 25%. The median survivals were reported to be in the range of 7 to 14.5 months. Compared with those reported regimens, the 36 Gy in three fractions in our dose-escalation study represents the highest fraction size patients have been treated with to date. In addition, the median tumor volume treated in this study (41.3 cc) is larger than the median tumor volume in the other studies. Despite these facts, the median survival in our study patients was 10 months, which was compatible with those outcomes previously reported. Of course, interstudy comparisons are always difficult because differences in patient selection criteria are applied in different centers. As noted earlier, two patients had a surgical resection of lesions that had enlarged following SRS, and in both cases, histopathology revealed extensive radionecrosis without tumor recurrence. Following these observations, later patients who had asymptomatic progression on MRI by conventional criteria were managed more conservatively. Gefitinib was continued unless there was intolerance to the agent or clinical deterioration. It should be noted that the RTOG central nervous system (CNS) toxicity criteria define clinically or radiographically suspected radionecrosis and histologically proved radionecrosis at the time of an operation as Grade 4 toxicity, whereas the CTC version 3 defines asymptomatic CNS necrosis with only radiographic findings as Grade 1 toxicity. Grade 2 CNS necrosis is defined as symptomatic but not interfering with active daily life (ADL). Grade 3 CNS necrosis is symptomatic and interferes with ADL. Grade 4 CNS necrosis is defined as life-threatening and requires operative intervention. In this study, two patients with pathologically proven necrosis were asymptomatic and therefore designated Grade 1. There is a clear need to improve local tumor control in high-grade gliomas because most patients die from progressive disease at or in close proximity to the primary site. To achieve local control, radiotherapy must be aggressive enough to kill the recurrent tumor, and there will likely be an unavoidable risk of tissue radionecrosis. Because of the poor prognosis of patients with recurrent high-grade gliomas, it is our opinion that asymptomatic radionecrosis should be an acceptable effect and thus not defined as a dose-limiting toxicity. Radionecrosis becomes an unacceptable consequence of radiotherapy only when it causes permanent neurologic deficit and adversely affects function and quality of life. Others have also noted that conventional MR imaging findings are frequently inadequate for reliably distinguishing radiation necrosis from tumor recurrence in patients with gliomas. Functional imaging can sometimes allow better distinction of recurrent tumor from radiation effects (30), although biopsy or surgical resection is still the gold standard for a definitive diagnosis Although the test has acknowledged limitations, the MMSE is at least somewhat useful to monitor cognitive function in patients who receive radiation to the brain. In six patients from our study, MMSE scores dramatically decreased to below the normal range (<27) shortly before disease progression was documented. The cause of cognitive decline seems to be related to disease progression. This is consistent with the growing evidence that the primary cause of cognitive decline in patients with brain tumors is tumor progression Concurrent chemotherapy with cisplatin, taxol, or topotecan had been used previously in conjunction with RT To date, these combined regimens have reportedly been well tolerated. Further, gefitinib has been reported to be well tolerated as a monotherapy, as well as to have modest activity in patients with recurrent glioblastoma. To our knowledge our study is the first to explore the combination of gefitinib and SRS. In summary, SRS to a total dose of 36 Gy in three fractions over 3 consecutive days is well tolerated with gefitinib at daily dose of 250 mg in the reirridiation setting. This SRS regimen requires minimal time commitment and inconvenience for the patients. Furthermore, this study demonstrated an encouraging overall survival rate compared with other reported series. The results support continued investigation of a new paradigm—namely, the combination of SRS and a novel agent targeting specific components of the tumor cell signal transduction pathway. Currently, in an ongoing follow-up clinical trial, fractionated SRS, using the dose established as safe (36 Gy in three fractions), is being combined with an agent that has broader activity in terms of its action to inhibit key molecular events in tumor growth and proliferation. |