| Predictors of Distant
Brain Recurrence for Patients With Newly Diagnosed Brain Metastases
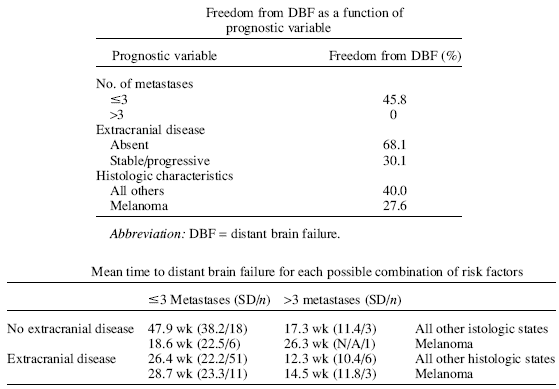
Treated With Stereotactic Radiosurgery Alone Stephen M. Sawrie, M.D., IJROBP Volume 70, Issue 1, Pages 181-186 (1 January 2008) To ascertain predictors of distant brain failure (DBF) in patients treated initially with stereotactic radiosurgery alone for newly diagnosed brain metastases. We hypothesize that these factors may be used to group patients according to risk of DBF. Methods and Materials We retrospectively analyzed 100 patients with newly diagnosed brain metastases treated from 2003 to 2005 at our Gamma Knife radiosurgery facility. The primary endpoint was DBF. Potential predictors included number of metastases, tumor volume, histologic characteristics, extracranial disease, and use of temozolomide. Results One-year actuarial risk of DBF was 61% for all patients. Significant predictors of DBF included more than three metastases (hazard ratio, 3.30; p = 0.004), stable or poorly controlled extracranial disease (hazard ratio, 2.16; p = 0.04), and melanoma histologic characteristics (hazard ratio, 2.14; p = 0.02). These were confirmed in multivariate analysis. Those with three or fewer metastases, no extracranial disease, and nonmelanoma histologic characteristics (N = 18) had a median time to DBF of 89 weeks vs. 33 weeks for all others. One-year actuarial freedom from DBF for this group was 83% vs. 26% for all others. Conclusions Independent significant predictors of DBF in our series included number of metastases (more than three), present or uncontrolled extracranial disease, and melanoma histologic characteristics. These factors were combined to identify a lower risk subgroup with significantly longer time to DBF. These patients may be candidates for initial localized treatment, reserving whole-brain radiation therapy for salvage. Patients in the higher risk group may be candidates for initial whole-brain radiation therapy or should be considered for clinical trials. |
|
|
|
Introduction Initial treatment of newly diagnosed brain metastases has become controversial. Traditionally, whole-brain radiation therapy (WBRT) has been the standard of care. In a study of various fractionation regimens, Borgelt reported 60–90% improvement in neurologic status, depending on the specific symptom. The greatest degree of improvement was seen in patients with severe baseline neurologic dysfunction, although improvement was documented in approximately two thirds of patients with only minimal neurologic sequelae. There was no clinical difference in fractionation scheme; therefore, the most common regimen of 30 Gy in 10 fractions is viewed as both efficacious and cost-effective. For example, Patchell showed that the addition of WBRT after surgical resection of a solitary metastasis improved local and distant brain failure (DBF) and decreased the probability of death secondary to neurologic causes. However, WBRT is not without toxicity. Acute side effects generally are tolerable and self-limited and include alopecia, fatigue, and headaches. The latter two symptoms are well managed with low-dose corticosteroids. However, late toxicities can be debilitating. DeAngelis profiled significant late toxicity in the absence of intracranial recurrence in 12 patients treated with WBRT to doses ranging from 25–36 Gy in 3–6-Gy fractions. All patients showed computed tomographic evidence of cortical atrophy and white matter abnormalities. Within a median of 14 weeks, all patients developed progressive dementia, ataxia, and urinary incontinence. Seven patients died as a result of these toxicities. Using a more sophisticated and empirical neurocognitive battery, Meyers reported neurologic and cognitive toxicities from a Phase III study of WBRT with and without motexafin gadolinium. All patients showed some level of neurocognitive decrease, although some of this was noted at baseline before treatment. More recently, stereotactic radiosurgery (SRS) was used with or without WBRT in the initial treatment of patients with newly diagnosed brain metastases. Recently, published data from a Phase III study of WBRT with or without an SRS boost showed significantly greater stability of function at 6 months in patients undergoing SRS as part of their initial treatment. Local control at 1 year improved from 71% to 82% with the addition of SRS. Subsequent subgroup analyses showed a survival advantage in patients with a solitary lesion, recursive partitioning analysis class I, or non–small cell histologic characteristics. In another recent Phase III trial, Aoyama compared outcomes in patients with one to four brain metastases treated initially with SRS and WBRT vs. SRS alone. There were no differences in overall survival or neurologic functional preservation, although local and distant brain control was greater with combined modalities. As a result, a greater percentage of patients treated with SRS alone eventually required salvage brain treatment. However, a retrospective analysis of patients who underwent SRS with or without WBRT showed no differences in local and distant brain control when accounting for subsequent salvage after first progression. These studies were used to support the omission of WBRT in the initial management of patients with one to four brain metastases. To date, no study attempted to specifically identify variables that would predict time to DBF (metachronous brain metastasis), although several studies included this in secondary analyses. Predictors of DBF from these studies included multiple brain metastases, active extracranial disease, total tumor volume, and time from diagnosis to development of brain metastases. The objective of this study therefore was to identify predictors of DBF in patients with newly diagnosed brain metastases treated initially with SRS alone. These variables may assist the clinician in identifying a subgroup of patients at low risk of developing DBF. This in turn would assist in deciding whether to reserve WBRT for salvage or include it in initial therapy. Discussion This study attempts to ascertain significant independent predictors of DBF in patients with newly diagnosed brain metastases treated initially with SRS alone in an attempt to better delineate whether to include WBRT as part of initial therapy. Univariate and multivariate analyses identified three variables that significantly and independently predicted DBF in this set of subjects. Stable or uncontrolled extracranial disease, more than three metastases, and melanoma histologic characteristics each predicted significantly shorter time to DBF. This is consistent with the previous literature. In a study of outcomes in 51 patients with melanoma histologic characteristics who underwent SRS alone for newly diagnosed brain metastases, the presence of extracranial metastases and multiple intracranial lesions predicted a shorter time to DBF. Lutterbach found that multiple metastases significantly predicted worse total brain freedom from progression (FFP) in patients with one to three lesions treated initially with SRS alone. Yu reported status of systemic disease as a significant predictor of DBF. In the recently published prospective study by Aoyama, multiple brain lesions, active extracranial disease, and Karnofsky performance scale (KPS) of 70–80 were associated significantly with the development of new brain metastases. One of the primary debates in the brain metastasis literature is whether to use SRS alone as initial therapy for patients with newly diagnosed brain metastases, reserving WBRT for salvage therapy. This debate prompted the American Society for Therapeutic Radiology and Oncology to publish an evidence-based review of the role of SRS as initial therapy for patients with brain metastases. The investigators applied randomized and retrospective literature to address key clinical questions of how using SRS alone as initial therapy affects survival, local control, distant intracranial control (i.e., DBF), and quality of life/symptom control. There now is clear evidence that the omission of WBRT from initial therapy for patients with brain metastases increases the risk of DBF. In Patchell , omission of WBRT after surgical resection of a solitary metastasis increased total brain failure (local plus DBF) from 18% to 70%. Sneed retrospectively reviewed 105 patients with one to four newly diagnosed brain metastases who underwent either SRS alone or SRS with WBRT. There were no differences in overall survival or 1-year local FFP, but 1-year total brain FFP (local plus distant) decreased from 69% to 28% with the omission of WBRT. Aoyama reported Phase III data for 132 patients with one to four brain metastases randomly assigned to SRS with or without WBRT. There was no survival or functional difference between arms. However, SRS alone increased DBF from 41.5% to 63.7% relative to the arm that also received WBRT. Whole-brain radiation therapy used as salvage can address the increased risk of DBF when using SRS alone as initial treatment. When allowing for salvage therapy, Sneed e found no difference in total brain control for patients undergoing SRS with or without WBRT (73% vs. 62%; p = 0.56). One of the primary advantages of using SRS alone is the neurocognitive protective effect that may be obtained by using WBRT at the time of local or distant intracranial relapse. However, neurocognitive effects of WBRT are poorly delineated. Extrapolating from studies of prophylactic cranial irradiation in patients with small-cell lung cancer, there does not appear to be significant short- or long-term cognitive sequelae. However, maximum doses and fraction sizes with prophylactic cranial irradiation regimens typically are smaller than with WBRT for brain metastases. DeAngelis were able to identify 12 patients from a review of 488 who underwent WBRT for brain metastases who had no evidence of intracranial recurrence, but who all developed significant progressive dementia. Fraction sizes in these 12 patients ranged from 3 to 6 Gy. Although the incidence of dementia in this series was low, consequences for those who developed this syndrome were debilitating. There is evidence of significant late cognitive effects 6 years after conformal radiation therapy for patients with low-grade gliomas. However, these doses typically exceeded 50 Gy. A randomized trial comparing a standard regimen of 30 Gy in 10 fractions to a regimen of 1.6 Gy twice daily to 54.4 Gy found a decrease of only 1.1 and 1.3 points on the Mini–Mental Status Examination, respectively. However, the Mini–Mental Status Examination is only a gross test of cognitive abilities and not very sensitive to even moderate cognitive decrease. There is stronger evidence to suggest that CNS progression after initial treatment for brain metastases negatively affects neurocognition. Regine reported outcomes in 36 patients who underwent SRS alone. Seventeen of these patients subsequently developed CNS progression (22% locally, 25% distantly). Of these 17 patients, 12 (71%) were symptomatic and 10 (59%) showed objective neurologic decrease at time of progression. In a Phase III study of WBRT with or without motexafin gadolinium, 401 patients underwent extensive neurocognitive evaluation before and after 30 Gy delivered in 10 fractions. In this study, 91% of the sample showed a significant decrease on at least one domain of cognition before treatment. Furthermore, neurocognitive decrease after treatment was associated most closely with tumor progression. In our cohort, we noted significant heterogeneity in the risk of DBF. However, patients without melanoma histologic characteristics, fewer than four metastases, and absent extracranial disease had a significantly lower risk of DBF. Median time to DBF for this low-risk subgroup was 89 weeks vs. 33 weeks for all others. One-year actuarial FDBF for the low-risk group was 83% vs. 26% for the remaining higher risk patients. These results may help delineate proper timing of WBRT in selected subsets of patients. It might be reasonable to treat these patients with SRS alone. These patients would still have the option of additional SRS or WBRT as salvage therapy depending on the nature and severity of any progression. Patients with such higher risk characteristics as four or more metastases, active extracranial disease, melanoma histologic characteristics, or any combination of these factors may be better candidates for WBRT as part of their initial treatment or for enrollment into clinical trials. Of course, results of this study are subject to any of the biases inherent in a retrospective analysis, and our risk stratification requires prospective validation. However, there are now multiple retrospective studies and one prospective study supporting the validity of these variables in predicting DBF. Their use in clinical trial stratification, as well as clinical practice, should further homogenize subsets of patients and further define appropriate initial management of newly diagnosed brain metastases. |