|
Multimodality treatment of melanoma brain metastases incorporating stereotactic radiosurgery (SRS) Wolfram E. Samlowski, Cancer 2007;109:1855 Brain metastases are a frequent
complication in advanced melanoma. A
3.6 to 4.1-month median
survival has been reported after treatment with whole brain
radiotherapy. We performed a retrospective analysis of our
institutional experience of multimodality treatment utilizing linear
accelerator (Linac)-based stereotactic radiosurgery (SRS).
Forty-four melanoma patients with brain metastases underwent 66 SRS
treatments for 156 metastatic foci between 1999 and 2004. Patients
were treated with initial
SRS if
|
|
|
| Annually, an estimated
97,000-170,000 cancer patients in the US develop brain metastases.
Melanoma ranks 4th overall as a cause of brain metastases (10% of
patients with brain metastases), after lung and breast cancer, and
unknown primary tumors, but is 2nd highest in incidence proportion
percentage. Approximately 10% to 13% of patients presenting with
regional disease (AJCC stage III) are at risk for brain metastases,
and 18% to 46% of stage IV patients will develop central nervous
system (CNS) involvement, with a prevalence of 55% to 75% at
autopsy. Factors that may associate with development of brain
metastases include male gender, mucosal or head and neck primaries,
as well as deep or ulcerated lesions. Development of brain
metastases leads directly to the patient's death in the majority of
cases. Current management strategies appear unsatisfactory,
and melanoma patients with brain metastases are usually excluded
from participation in clinical trials due to a general perception of
an adverse prognosis. Surgical
resection, usually followed by radiotherapy, has been employed to
treat brain metastases in selected patients (usually with solitary,
superficial lesions). Extended survival can be achieved in patients
with high performance status or Radiation Therapy Oncology Group (RTOG)
Recursive Partition Analysis (RPA) Class I (Karnofsky Performance
Status [KPS]
A series of recent publications have suggested high local control rates for brain metastases with stereotactic radiosurgery (SRS), using either linear accelerator (Linac)- or Gamma-Knife-based approaches. In these studies SRS has generally been limited to patients with 1 to 3 metastases, with rare series accepting patients with larger numbers of lesions. We report our encouraging institutional experience, based on expanded eligibility for Linac-based SRS for patients with up to 5 brain metastases, followed by planned systemic therapy. Patients with advanced melanoma
develop brain metastases with such a high frequency that development
of appropriate screening and management strategies represents an
important clinical challenge. What appears extremely clear is that
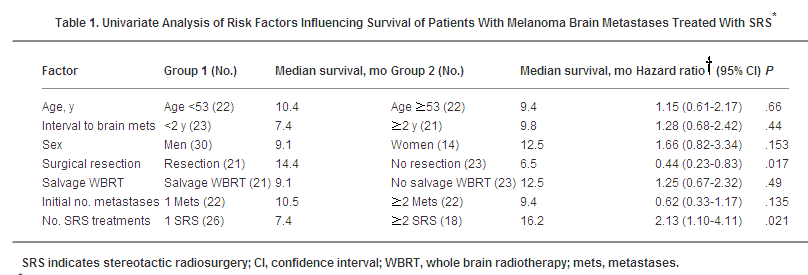
nontreatment of melanoma brain metastases ( We used SRS as the primary treatment for 44 sequential patients with melanoma brain metastases, using a multimodality treatment algorithm. Subsequent systemic therapy was also planned if metastatic disease was not limited to the CNS. In our series half of the patients presented with a solitary brain metastasis. The other half had multiple metastases. Our results indicate that the vast majority of patients with brain metastases from melanoma can be effectively treated using SRS as the mainstay of therapy. SRS demonstrated a high local control rate, with median duration of response in treated lesions lasting 10.2 months and a prolonged median survival (11.1 months from diagnosis). We found that additional SRS treatments, surgical resection, and WBRT could be employed as an adjunct to initial SRS treatment to maintain control of brain metastases. Exploratory Cox univariate analysis suggested that surgery and multiple SRS treatments appeared to correlate with prolonged survival. Furthermore, 81% of patients with concurrent non-CNS metastases tolerated subsequent systemic treatment without undue toxicity. Although not quantified, quality of life and neurologic function appeared well maintained for protracted periods. The SRS treatment approach was based on recent publications suggesting high local control rates for brain metastases treated with SRS using either Linac- or Gamma-Knife-based approaches. In a melanoma-specific patient series, Gonzales-Martinez treated 24 patients with 115 lesions with Gamma-Knife (mean radiotherapy dose 16.4 Gy), with a median survival of 5.5 months after radiosurgery. Median tumor volume treated was 4 cm3. Noel treated 25 patients with 61 melanoma brain metastases using invasive immobilization and Linac-based radiosurgery (mostly for 1-3 metastases). The median dose was 17 Gy and local control was achieved in 81% at 1 year. Median survival was 8 months, with 29% 1-year survival. Herfarth employed Linac stereotactic radiotherapy in 64 patients with 122 melanoma brain metastases, with a median dose of 20 Gy. CNS control was achieved in 81% and median survival was 10.6 months. Radbill treated 188 melanoma brain metastases in 51 patients using a Gamma-Knife, achieving 81% local control in the CNS. A 17.7-month survival was achieved in patients with a solitary metastasis, compared with 4.6 months for patients with multiple metastases. RPA class I patients had a median survival of 57 weeks vs 20 weeks for RPA class II or III. The majority of patients had additional brain metastases develop. Selek reported the results of Linac-based SRS in 103 patients with 153 brain metastases. The median tumor volume treated was 1.8 cm3, with a median tumor dose of 18 Gy. Local control of treated lesion was 49% and CNS progression-free survival was 15% at 1 year. Overall survival was 25% at 1 year. Koc treated 26 melanoma patients with 72 brain metastases using Gamma-Knife. The median survival was 9 months from diagnosis and 1 year survival was 25%. Recently, Gaudy-Marqueste updated results of Gamma-Knife treatment of 1 to 4 melanoma brain metastases. These investigators treated 221 brain metastases in 106 patients (61.3% were solitary metastases). A high local control rate (83.7) was observed, but median survival was found to be only 5.1 months. A number of conclusions can be drawn from these studies, in conjunction with our own data: SRS or Gamma-Knife treatment appears to produce high control rates in patients with small numbers of brain metastases and good performance status. Some of these studies, including our own, also demonstrated prolonged progression-free and overall survival. In contrast to some published series, single brain metastases and use of additional WBRT did not confer survival benefit. Because all surviving patients in the
current series were followed for a minimum of 1 year after SRS, we
can clearly define causes of treatment failure. Six (14%) patients
currently remain alive in remission or continue to respond to
therapy (only 1 of these initially presented with a CNS-only
recurrence, the rest had systemic metastatic disease). Of the 38
patients who died, 6 (14%) died of systemic progression only, 11
(25%) died with concurrent systemic and CNS, and 21 (48%) patients
died predominantly of CNS progression. These results indicate that,
whereas SRS provides lengthy palliation, maintenance of quality of
life and prolongation of survival, further improvement in CNS
control is needed, as progression at treated sites and development
of new brain metastases contributed to eventual death in
Whether SRS treatment can be used
without routine addition of WBRT is currently being evaluated. In a
series of renal cancer, melanoma and sarcoma patients treated for 1
to 3 brain metastases using radiosurgery by the Eastern Cooperative
Oncology Group, intracranial failure rates were 25.8% and 48.3% at 3
and 6 months, respectively. Failure rates within and outside the
treatment volume appeared similar. This translated into a median
survival of 8.3 months. It is difficult to analyze the impact of SRS
in melanoma patients, however, because this was not separately
reported. Still, those authors concluded that the intracranial
failure rate suggested that routine avoidance of WBRT should be
approached judiciously. A recent randomized study by Aoyama
demonstrated that addition of WBRT to SRS did not significantly
improve survival in patients with 1 to 4 brain metastases (mostly
lung cancer), with a median survival of 8.0 months with SRS alone,
vs 7.5 months with SRS + WBRT. CNS recurrences were seen in 29 of 67
patients after SRS alone, compared with 10 of 65 patients treated
with SRS + WBRT. This resulted in frequent addition of salvage WBRT
in the SRS-only group. It is clear from our study and that of Aoyama
et al that salvage therapy after SRS is feasible without adverse
effects on overall survival. The advantage of this approach is
avoidance of any unnecessary CNS toxicity from additional
radiotherapy (SRS or WBRT) in approximately 50% of patients. The
question of whether SRS alone is adequate initial treatment for
patients with
In conclusion, we report an encouraging institutional experience treating melanoma brain metastases, based on expanded eligibility for Linac-based SRS for patients with up to 5 brain metastases. Based on our results, we propose the following treatment algorithm. SRS treatment of melanoma brain metastases followed by systemic treatment provides appears to provide useful palliation and prolonged survival. Due to improvements in linear accelerator design and computerized treatment planning software, even more complex SRS treatment planning is currently possible. Based on the these results, exclusion of appropriately treated patients with melanoma brain metastases from clinical trials does not appear warranted because survival is similar to stage IV melanoma overall. There remains a need to develop more active agents for treatment of metastatic melanoma, with particular emphasis on drugs that penetrate into the CNS to reduce the high incidence of brain metastases. |
 5
brain metastases were present
5
brain metastases were present 70,
but 37 patients had active systemic metastases (Recursive Partition
Analysis Class 2). Dose was prescribed to the isodose line, which
covered
70,
but 37 patients had active systemic metastases (Recursive Partition
Analysis Class 2). Dose was prescribed to the isodose line, which
covered
 palliative
care
palliative
care )
results in rapid patient decline and death. Surgical
resection, usually followed by radiotherapy, has been employed to
treat brain metastases in selected patients (generally solitary
metastases in a superficial location). These patients may have
extended survival, particularly if they have a high performance
status or RTOG RPA class I (KPS >70%, no synchronous systemic
metastases). Most patients with melanoma brain metastases are
not considered surgical candidates. In these patients WBRT
(generally 3000 cGy in 10 fractions) has been widely employed to
palliate brain metastases, resulting in 3.6 to 4.8-month median
survival. Performance status and RTOG RPA class are important
determinants of survival after WBRT.
)
results in rapid patient decline and death. Surgical
resection, usually followed by radiotherapy, has been employed to
treat brain metastases in selected patients (generally solitary
metastases in a superficial location). These patients may have
extended survival, particularly if they have a high performance
status or RTOG RPA class I (KPS >70%, no synchronous systemic
metastases). Most patients with melanoma brain metastases are
not considered surgical candidates. In these patients WBRT
(generally 3000 cGy in 10 fractions) has been widely employed to
palliate brain metastases, resulting in 3.6 to 4.8-month median
survival. Performance status and RTOG RPA class are important
determinants of survival after WBRT. 73%
of patients.
73%
of patients.